200,000+ products from a single source!
sales@angenechem.com
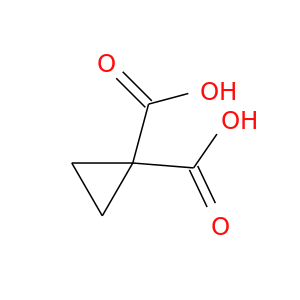
139781-05-8 | Cyclopropanedicarboxylic acid
CAS No: 139781-05-8 Catalog No: AG001BOZ MDL No:
Product Description
Catalog Number:
AG001BOZ
Chemical Name:
Cyclopropanedicarboxylic acid
CAS Number:
139781-05-8
Molecular Formula:
C5H6O4
Molecular Weight:
130.0987
IUPAC Name:
cyclopropane-1,1-dicarboxylic acid
InChI:
InChI=1S/C5H6O4/c6-3(7)5(1-2-5)4(8)9/h1-2H2,(H,6,7)(H,8,9)
InChI Key:
FDKLLWKMYAMLIF-UHFFFAOYSA-N
SMILES:
OC(=O)C1(CC1)C(=O)O
EC Number:
209-917-2
NSC Number:
626865
Properties
Complexity:
152
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
130.027g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
130.099g/mol
Monoisotopic Mass:
130.027g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
74.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.2
Literature
| Title | Journal |
|---|---|
| Metal-chelating polymers by anionic ring-opening polymerization and their use in quantitative mass cytometry. | Biomacromolecules 20120813 |
| Self-assembly of amphiphilic liquid crystal polymers obtained from a cyclopropane-1,1-dicarboxylate bearing a cholesteryl mesogen. | Langmuir : the ACS journal of surfaces and colloids 20120731 |
| Protocol: An updated integrated methodology for analysis of metabolites and enzyme activities of ethylene biosynthesis. | Plant methods 20110101 |
| Binding of flexible and constrained ligands to the Grb2 SH2 domain: structural effects of ligand preorganization. | Acta crystallographica. Section D, Biological crystallography 20101001 |
| trans-Directing ability of the amide group: enabling the enantiocontrol in the synthesis of 1,1-dicarboxy cyclopropanes. Reaction development, scope, and synthetic applications. | The Journal of organic chemistry 20091204 |
| Expedient synthesis of pyrrolo[1,2-a]indoles: preparation of the core of yuremamine. | Organic letters 20080821 |
| Discovery of novel hydroxamates as highly potent tumor necrosis factor-alpha converting enzyme inhibitors: Part I--discovery of two binding modes. | Journal of medicinal chemistry 20080227 |
| Scandium triflate catalyzed cycloaddition of imines with 1,1-cyclopropanediesters: efficient and diastereoselective synthesis of multi-substituted pyrrolidines. | Organic & biomolecular chemistry 20060121 |
| Synthesis of thiadiazoles and 1,2,4-triazoles derived from cyclopropane dicarboxylic acid. | Molecules (Basel, Switzerland) 20050930 |
| The simultaneous determination of 1-aminocyclopropane-1-carboxylic acid and cyclopropane-1,1-dicarboxylic acid in Lycopersicum esculentum by high-performance liquid chromatography--electrospray tandem mass spectrometry. | Phytochemical analysis : PCA 20030101 |
| Prime site binding inhibitors of a serine protease: NS3/4A of hepatitis C virus. | Biochemistry 20020430 |
Related Products
© 2019 Angene International Limited. All rights Reserved.