200,000+ products from a single source!
sales@angenechem.com
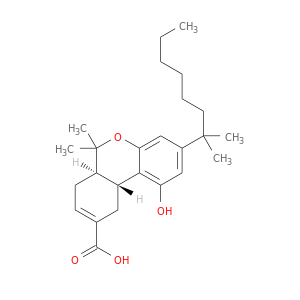
137945-48-3 | 6H-Dibenzo[b,d]pyran-9-carboxylic acid, 3-(1,1-dimethylheptyl)-6a,7,10,10a-tetrahydro-1-hydroxy-6,6-dimethyl-, (6aR,10aR)-
CAS No: 137945-48-3 Catalog No: AG0017PV MDL No:
Product Description
Catalog Number:
AG0017PV
Chemical Name:
6H-Dibenzo[b,d]pyran-9-carboxylic acid, 3-(1,1-dimethylheptyl)-6a,7,10,10a-tetrahydro-1-hydroxy-6,6-dimethyl-, (6aR,10aR)-
CAS Number:
137945-48-3
Molecular Formula:
C25H36O4
Molecular Weight:
400.5509
IUPAC Name:
(6aR,10aR)-1-hydroxy-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c]chromene-9-carboxylic acid
InChI:
InChI=1S/C25H36O4/c1-6-7-8-9-12-24(2,3)17-14-20(26)22-18-13-16(23(27)28)10-11-19(18)25(4,5)29-21(22)15-17/h10,14-15,18-19,26H,6-9,11-13H2,1-5H3,(H,27,28)/t18-,19-/m1/s1
InChI Key:
YCHYFHOSGQABSW-RTBURBONSA-N
SMILES:
CCCCCCC(c1cc(O)c2c(c1)OC([C@H]1[C@H]2CC(=CC1)C(=O)O)(C)C)(C)C
UNII:
OGN7X90BT8
Properties
Complexity:
620
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
0
Exact Mass:
400.261g/mol
Formal Charge:
0
Heavy Atom Count:
29
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
400.559g/mol
Monoisotopic Mass:
400.261g/mol
Rotatable Bond Count:
7
Topological Polar Surface Area:
66.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
7
Literature
| Title | Journal |
|---|---|
| Ajulemic acid: potential treatment for chronic inflammation. | Pharmacology research & perspectives 20180401 |
| Therapeutic utility of cannabinoid receptor type 2 (CB(2)) selective agonists. | Journal of medicinal chemistry 20131114 |
| Synthetic cannabinoid ajulemic acid exerts potent antifibrotic effects in experimental models of systemic sclerosis. | Annals of the rheumatic diseases 20120901 |
| Lack of positive allosteric modulation of mutated alpha(1)S267I glycine receptors by cannabinoids. | Naunyn-Schmiedeberg's archives of pharmacology 20100501 |
| Ajulemic acid, a synthetic cannabinoid, increases formation of the endogenous proresolving and anti-inflammatory eicosanoid, lipoxin A4. | FASEB journal : official publication of the Federation of American Societies for Experimental Biology 20090501 |
| Positive allosteric modulatory effects of ajulemic acid at strychnine-sensitive glycine alpha1- and alpha1beta-receptors. | Naunyn-Schmiedeberg's archives of pharmacology 20090401 |
| Cannabinoids, endocannabinoids, and related analogs in inflammation. | The AAPS journal 20090301 |
| Acylamido analogs of endocannabinoids selectively inhibit cancer cell proliferation. | Bioorganic & medicinal chemistry 20081115 |
| Ajulemic acid, a synthetic cannabinoid acid, induces an antiinflammatory profile of eicosanoids in human synovial cells. | Life sciences 20081107 |
| Functional and immunohistochemical characterization of CB1 and CB2 receptors in rat bladder. | Urology 20081101 |
| Suppression of human macrophage interleukin-6 by a nonpsychoactive cannabinoid acid. | Rheumatology international 20080501 |
| Ajulemic acid, a nonpsychoactive cannabinoid acid, suppresses osteoclastogenesis in mononuclear precursor cells and induces apoptosis in mature osteoclast-like cells. | Journal of cellular physiology 20080301 |
| Comments on 'cannabimimetic properties of ajulemic acid'. | The Journal of pharmacology and experimental therapeutics 20070701 |
| Effects of IP-751, ajulemic acid, on bladder overactivity induced by bladder irritation in rats. | Urology 20070701 |
| Ajulemic acid, a synthetic nonpsychoactive cannabinoid acid, bound to the ligand binding domain of the human peroxisome proliferator-activated receptor gamma. | The Journal of biological chemistry 20070622 |
| Cannabimimetic properties of ajulemic acid. | The Journal of pharmacology and experimental therapeutics 20070201 |
| Suppression of fibroblast metalloproteinases by ajulemic acid, a nonpsychoactive cannabinoid acid. | Journal of cellular biochemistry 20070101 |
| Ajulemic acid. | IDrugs : the investigational drugs journal 20051201 |
| PPAR-gamma: a nuclear receptor with affinity for cannabinoids. | Life sciences 20050819 |
| Effect of the cannabinoid ajulemic acid on rat models of neuropathic and inflammatory pain. | Neuroscience letters 20050715 |
| Antihyperalgesic properties of the cannabinoid CT-3 in chronic neuropathic and inflammatory pain states in the rat. | Pain 20050701 |
| Determination of ajulemic acid and its glucuronide in human plasma by gas chromatography-mass spectrometry. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20050605 |
| Pain measurements and side effect profile of the novel cannabinoid ajulemic acid. | Neuropharmacology 20050601 |
| Ajulemic acid (IP-751): synthesis, proof of principle, toxicity studies, and clinical trials. | The AAPS journal 20050301 |
| Pain reduction and lack of psychotropic effects with ajulemic acid: comment on the article by Sumariwalla et al. | Arthritis and rheumatism 20041201 |
| Ajulemic acid: A novel cannabinoid produces analgesia without a 'high'. | Life sciences 20040806 |
| A novel synthetic, nonpsychoactive cannabinoid acid (HU-320) with antiinflammatory properties in murine collagen-induced arthritis. | Arthritis and rheumatism 20040301 |
| Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: a randomized controlled trial. | JAMA 20031001 |
| Ajulemic acid, a nonpsychoactive cannabinoid acid, induces apoptosis in human T lymphocytes. | Clinical immunology (Orlando, Fla.) 20030801 |
| Activation and binding of peroxisome proliferator-activated receptor gamma by synthetic cannabinoid ajulemic acid. | Molecular pharmacology 20030501 |
| Suppression of human monocyte interleukin-1beta production by ajulemic acid, a nonpsychoactive cannabinoid. | Biochemical pharmacology 20030215 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







