200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 137201-62-8
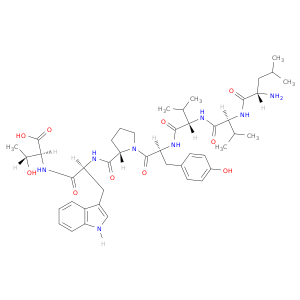
137201-62-8 | L-Threonine, L-leucyl-L-valyl-L-valyl-L-tyrosyl-L-prolyl-L-tryptophyl-
CAS No: 137201-62-8 Catalog No: AG0012XS MDL No:
Product Description
Catalog Number:
AG0012XS
Chemical Name:
L-Threonine, L-leucyl-L-valyl-L-valyl-L-tyrosyl-L-prolyl-L-tryptophyl-
CAS Number:
137201-62-8
Molecular Formula:
C45H64N8O10
Molecular Weight:
877.0373
IUPAC Name:
(2S,3R)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-4-methylpentanoyl]amino]-3-methylbutanoyl]amino]-3-methylbutanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]pyrrolidine-2-carbonyl]amino]-3-(1H-indol-3-yl)propanoyl]amino]-3-hydroxybutanoic acid
InChI:
InChI=1S/C45H64N8O10/c1-23(2)19-31(46)39(56)50-37(25(5)6)43(60)51-36(24(3)4)42(59)49-34(20-27-14-16-29(55)17-15-27)44(61)53-18-10-13-35(53)41(58)48-33(40(57)52-38(26(7)54)45(62)63)21-28-22-47-32-12-9-8-11-30(28)32/h8-9,11-12,14-17,22-26,31,33-38,47,54-55H,10,13,18-21,46H2,1-7H3,(H,48,58)(H,49,59)(H,50,56)(H,51,60)(H,52,57)(H,62,63)/t26-,31+,33+,34+,35+,36+,37+,38+/m1/s1
InChI Key:
BXIFNVGZIMFBQB-DYDSHOKNSA-N
SMILES:
CC(C[C@@H](C(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@H](C(=O)N1CCC[C@H]1C(=O)N[C@H](C(=O)N[C@H](C(=O)O)[C@H](O)C)Cc1c[nH]c2c1cccc2)Cc1ccc(cc1)O)C(C)C)C(C)C)N)C
Properties
Complexity:
1590
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
8
Defined Bond Stereocenter Count:
0
Exact Mass:
876.475g/mol
Formal Charge:
0
Heavy Atom Count:
63
Hydrogen Bond Acceptor Count:
11
Hydrogen Bond Donor Count:
10
Isotope Atom Count:
0
Molecular Weight:
877.053g/mol
Monoisotopic Mass:
876.475g/mol
Rotatable Bond Count:
21
Topological Polar Surface Area:
285A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1
Literature
| Title | Journal |
|---|---|
| Structure-activity relationship studies of spinorphin as a potent and selective human P2X(3) receptor antagonist. | Journal of medicinal chemistry 20070906 |
| Spinorphin as an endogenous inhibitor of enkephalin-degrading enzymes: roles in pain and inflammation. | Current protein & peptide science 20021201 |
| The endogenous opioid spinorphin blocks fMet-Leu-Phe-induced neutrophil chemotaxis by acting as a specific antagonist at the N-formylpeptide receptor subtype FPR. | Journal of immunology (Baltimore, Md. : 1950) 20011201 |
| Spinorphin, an endogenous inhibitor of enkephalin-degrading enzymes, potentiates leu-enkephalin-induced anti-allodynic and antinociceptive effects in mice. | Japanese journal of pharmacology 20011201 |
| Identification of dipeptidyl peptidase III in human neutrophils. | Biochemical and biophysical research communications 20000705 |
| Inhibitory effects of spinorphin, a novel endogenous regulator, on chemotaxis, O2- generation, and exocytosis by N-formylmethionyl-leucyl-phenylalanine (FMLP)-stimulated neutrophils. | Biochemical pharmacology 19970915 |
| Reversed-phase high-performance liquid chromatography for the determination of haemorphin-like immunoreactivity in human cerebrospinal fluid. | Journal of chromatography. A 19940729 |
| A structural homologue of the N-formyl peptide receptor. Characterization and chromosome mapping of a peptide chemoattractant receptor family. | The Journal of biological chemistry 19920415 |
Related Products
© 2019 Angene International Limited. All rights Reserved.