200,000+ products from a single source!
sales@angenechem.com
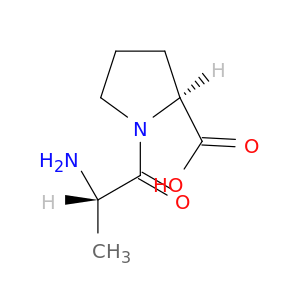
13485-59-1 | (S)-1-((S)-2-Aminopropanoyl)pyrrolidine-2-carboxylic acid
CAS No: 13485-59-1 Catalog No: AG003CIM MDL No:MFCD00037332
Product Description
Catalog Number:
AG003CIM
Chemical Name:
(S)-1-((S)-2-Aminopropanoyl)pyrrolidine-2-carboxylic acid
CAS Number:
13485-59-1
Molecular Formula:
C8H14N2O3
Molecular Weight:
186.2084
MDL Number:
MFCD00037332
IUPAC Name:
(2S)-1-[(2S)-2-aminopropanoyl]pyrrolidine-2-carboxylic acid
InChI:
InChI=1S/C8H14N2O3/c1-5(9)7(11)10-4-2-3-6(10)8(12)13/h5-6H,2-4,9H2,1H3,(H,12,13)/t5-,6-/m0/s1
InChI Key:
WPWUFUBLGADILS-WDSKDSINSA-N
SMILES:
OC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C
EC Number:
236-795-8
Properties
Complexity:
229
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
0
Exact Mass:
186.1g/mol
Formal Charge:
0
Heavy Atom Count:
13
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
186.211g/mol
Monoisotopic Mass:
186.1g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
83.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-2.7
Literature
| Title | Journal |
|---|---|
| Moisture-Absorption and Water Dynamics in the Powder of Egg Albumen Peptide, Met-Pro-Asp-Ala-His-Leu. | Journal of food science 20170101 |
| Fragmental modeling of hPepT2 and analysis of its binding features by docking studies and pharmacophore mapping. | Bioorganic & medicinal chemistry 20110801 |
| Conformational preferences of X-Pro sequences: Ala-Pro and Aib-Pro motifs. | The journal of physical chemistry. B 20101111 |
| Activity of prolidase isoenzymes in the rat brain: subcellular and regional distribution during development. | Brain research 20091215 |
| Species-specific differences in the Pro-Ala rich region of cardiac myosin binding protein-C. | Journal of muscle research and cell motility 20091201 |
| Purification, characterization, and gene cloning of glucose-1-phosphatase from Citrobacter braakii. | The Journal of general and applied microbiology 20091001 |
| Comparison between integrated and parallel tempering methods in enhanced sampling simulations. | The Journal of chemical physics 20090328 |
| Charge-based interactions between peptides observed as the dominant force for association in aqueous solution. | Angewandte Chemie (International ed. in English) 20080101 |
| Conjugation of poorly absorptive drugs with mucoadhesive polymers for the improvement of oral absorption of drugs. | Journal of controlled release : official journal of the Controlled Release Society 20071120 |
| Prolidase isoenzymes in the rat: their organ distribution, developmental change and specific inhibitors. | Pediatric research 20070701 |
| A molecular dynamics study of Cyclophilin A free and in complex with the Ala-Pro dipeptide. | European biophysics journal : EBJ 20070301 |
| Stereoselective synthesis of (Z)-alkene-containing proline dipeptide mimetics. | The Journal of organic chemistry 20060623 |
| Automated method, based on micro-sequential injection, for the study of enzyme kinetics and inhibition. | Analytical sciences : the international journal of the Japan Society for Analytical Chemistry 20060101 |
| Enhanced tumor cell selectivity of adriamycin-monoclonal antibody conjugate via a poly(ethylene glycol)-based cleavable linker. | Journal of controlled release : official journal of the Controlled Release Society 20020219 |
| Determination of the cis-trans isomerization barrier of several L-peptidyl-L-proline dipeptides by dynamic capillary electrophoresis and computer simulation. | Electrophoresis 20010801 |
| Chirality organization of ferrocenes bearing podand dipeptide chains: synthesis and structural characterization. | Journal of the American Chemical Society 20010110 |
| Inhibitors of tripeptidyl peptidase II. 2. Generation of the first novel lead inhibitor of cholecystokinin-8-inactivating peptidase: a strategy for the design of peptidase inhibitors. | Journal of medicinal chemistry 20000224 |
Related Products
© 2019 Angene International Limited. All rights Reserved.