200,000+ products from a single source!
sales@angenechem.com
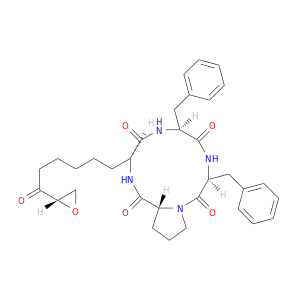
133155-90-5 | Cyclo[(αS,2S)-α-amino-η-oxo-2-oxiraneoctanoyl-L-phenylalanyl-L-phenylalanyl-D-prolyl]
CAS No: 133155-90-5 Catalog No: AG0014CR MDL No:
Product Description
Catalog Number:
AG0014CR
Chemical Name:
Cyclo[(αS,2S)-α-amino-η-oxo-2-oxiraneoctanoyl-L-phenylalanyl-L-phenylalanyl-D-prolyl]
CAS Number:
133155-90-5
Molecular Formula:
C33H40N4O6
Molecular Weight:
588.6939
IUPAC Name:
(3S,6S,9S,12R)-3,6-dibenzyl-9-[6-[(2S)-oxiran-2-yl]-6-oxohexyl]-1,4,7,10-tetrazabicyclo[10.3.0]pentadecane-2,5,8,11-tetrone
InChI:
InChI=1S/C33H40N4O6/c38-28(29-21-43-29)17-9-3-8-15-24-30(39)35-25(19-22-11-4-1-5-12-22)31(40)36-26(20-23-13-6-2-7-14-23)33(42)37-18-10-16-27(37)32(41)34-24/h1-2,4-7,11-14,24-27,29H,3,8-10,15-21H2,(H,34,41)(H,35,39)(H,36,40)/t24-,25-,26-,27+,29-/m0/s1
InChI Key:
LLOKIGWPNVSDGJ-AFBVCZJXSA-N
SMILES:
O=C1N[C@@H](Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N2[C@@H](C(=O)N[C@H]1CCCCCC(=O)[C@@H]1CO1)CCC2
NSC Number:
700657
Properties
Complexity:
1010
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
5
Defined Bond Stereocenter Count:
0
Exact Mass:
588.295g/mol
Formal Charge:
0
Heavy Atom Count:
43
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
588.705g/mol
Monoisotopic Mass:
588.295g/mol
Rotatable Bond Count:
11
Topological Polar Surface Area:
137A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.1
Literature
| Title | Journal |
|---|---|
| Design and synthesis of novel hybrid benzamide-peptide histone deacetylase inhibitors. | Bioorganic & medicinal chemistry letters 20090715 |
| Splicing factor SF3b as a target of the antitumor natural product pladienolide. | Nature chemical biology 20070901 |
| Effect of inhibitors of histone deacetylase on the induction of cell differentiation in murine and human erythroleukemia cell lines. | Anti-cancer drugs 20050701 |
| Class II (IIa)-selective histone deacetylase inhibitors. 1. Synthesis and biological evaluation of novel (aryloxopropenyl)pyrrolyl hydroxyamides. | Journal of medicinal chemistry 20050505 |
| Antiproliferative and phenotype-transforming antitumor agents derived from cysteine. | Journal of medicinal chemistry 20040603 |
| N-hydroxy-3-phenyl-2-propenamides as novel inhibitors of human histone deacetylase with in vivo antitumor activity: discovery of (2E)-N-hydroxy-3-[4-[[(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2-propenamide (NVP-LAQ824). | Journal of medicinal chemistry 20031009 |
| Inhibitors of human histone deacetylase: synthesis and enzyme and cellular activity of straight chain hydroxamates. | Journal of medicinal chemistry 20020214 |
| 3-(4-aroyl-1H-pyrrol-2-yl)-N-hydroxy-2-propenamides, a new class of synthetic histone deacetylase inhibitors. | Journal of medicinal chemistry 20010621 |
Related Products
© 2019 Angene International Limited. All rights Reserved.