200,000+ products from a single source!
sales@angenechem.com
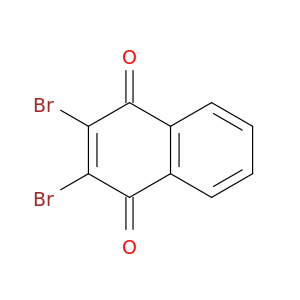
13243-65-7 | 1,4-Naphthalenedione, 2,3-dibromo-
CAS No: 13243-65-7 Catalog No: AG0010OQ MDL No:
Product Description
Catalog Number:
AG0010OQ
Chemical Name:
1,4-Naphthalenedione, 2,3-dibromo-
CAS Number:
13243-65-7
Molecular Formula:
C10H4Br2O2
Molecular Weight:
315.9456
IUPAC Name:
2,3-dibromonaphthalene-1,4-dione
InChI:
InChI=1S/C10H4Br2O2/c11-7-8(12)10(14)6-4-2-1-3-5(6)9(7)13/h1-4H
InChI Key:
PSMABVOYZJWFBV-UHFFFAOYSA-N
SMILES:
O=C1C(=C(Br)C(=O)c2c1cccc2)Br
EC Number:
236-223-7
Properties
Complexity:
301
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
315.856g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
315.948g/mol
Monoisotopic Mass:
313.858g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
34.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.8
Literature
| Title | Journal |
|---|---|
| Analogs of N'-hydroxy-N-(4H,5H-naphtho[1,2-d]thiazol-2-yl)methanimidamide inhibit Mycobacterium tuberculosis methionine aminopeptidases. | Bioorganic & medicinal chemistry 20120715 |
| Synthesis and antioxidant evaluation of some new pyrazolopyridine derivatives. | Archiv der Pharmazie 20120201 |
| Leishmanicidal activity of two naphthoquinones against Leishmania donovani. | Biological & pharmaceutical bulletin 20120101 |
| Cytotoxicity of naphthoquinones and their capacity to generate reactive oxygen species is quenched when conjugated with gold nanoparticles. | International journal of nanomedicine 20110101 |
| Acaricidal activity and function of mite indicator using plumbagin and its derivatives isolated from Diospyros kaki Thunb. roots (Ebenaceae). | Journal of microbiology and biotechnology 20080201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







