200,000+ products from a single source!
sales@angenechem.com
Home > Indoles and Oxindole > 13228-40-5
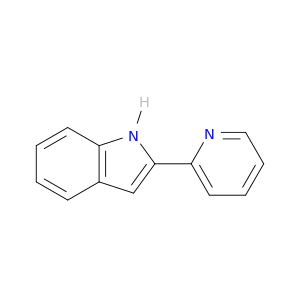
13228-40-5 | 1H-Indole, 2-(2-pyridinyl)-
CAS No: 13228-40-5 Catalog No: AG0010HJ MDL No:MFCD00033466
Product Description
Catalog Number:
AG0010HJ
Chemical Name:
1H-Indole, 2-(2-pyridinyl)-
CAS Number:
13228-40-5
Molecular Formula:
C13H10N2
Molecular Weight:
194.2319
MDL Number:
MFCD00033466
IUPAC Name:
2-pyridin-2-yl-1H-indole
InChI:
InChI=1S/C13H10N2/c1-2-6-11-10(5-1)9-13(15-11)12-7-3-4-8-14-12/h1-9,15H
InChI Key:
OLGGLCIDAMICTA-UHFFFAOYSA-N
SMILES:
c1ccc(nc1)c1cc2c([nH]1)cccc2
NSC Number:
112668
Properties
Complexity:
217
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
194.084g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
194.237g/mol
Monoisotopic Mass:
194.084g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
28.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.3
Literature
| Title | Journal |
|---|---|
| Neutral cuprous complexes as ratiometric oxygen gas sensors. | Dalton transactions (Cambridge, England : 2003) 20120128 |
| Steric and electronic influence on photochromic switching of N,C-chelate four-coordinate organoboron compounds. | Chemistry (Weinheim an der Bergstrasse, Germany) 20100426 |
| Isocyanate-, isothiocyanate-, urea-, and thiourea-substituted boron dipyrromethene dyes as fluorescent probes. | The Journal of organic chemistry 20060414 |
| 2-Pyridin-2-yl-1H-indole derivatives: synthesis, estrogen receptor binding affinity, and photophysical properties. | Bioorganic chemistry 20060201 |
| Parallel synthesis of 5-cyano-6-aryl-2-thiouracil derivatives as inhibitors for hepatitis C viral NS5B RNA-dependent RNA polymerase. | Bioorganic chemistry 20060201 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.