200,000+ products from a single source!
sales@angenechem.com
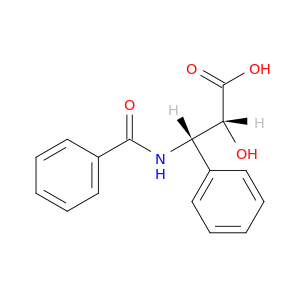
132201-33-3 | Benzenepropanoic acid, β-(benzoylamino)-α-hydroxy-, (αR,βS)-
CAS No: 132201-33-3 Catalog No: AG0010DS MDL No:MFCD00274633
Product Description
Catalog Number:
AG0010DS
Chemical Name:
Benzenepropanoic acid, β-(benzoylamino)-α-hydroxy-, (αR,βS)-
CAS Number:
132201-33-3
Molecular Formula:
C16H15NO4
Molecular Weight:
285.2946
MDL Number:
MFCD00274633
IUPAC Name:
(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoic acid
InChI:
InChI=1S/C16H15NO4/c18-14(16(20)21)13(11-7-3-1-4-8-11)17-15(19)12-9-5-2-6-10-12/h1-10,13-14,18H,(H,17,19)(H,20,21)/t13-,14+/m0/s1
InChI Key:
HYJVYOWKYPNSTK-UONOGXRCSA-N
SMILES:
O=C(c1ccccc1)N[C@H]([C@H](C(=O)O)O)c1ccccc1
UNII:
D998Q2WJCM
Properties
Complexity:
359
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
0
Exact Mass:
285.1g/mol
Formal Charge:
0
Heavy Atom Count:
21
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
285.299g/mol
Monoisotopic Mass:
285.1g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
86.6A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.7
Literature
| Title | Journal |
|---|---|
| Phenylisoserine in the gas-phase and water: Ab initio studies on neutral and zwitterion conformers. | Journal of molecular modeling 20110501 |
| Clinical trials and progress with paclitaxel in ovarian cancer. | International journal of women's health 20100101 |
| Cytotoxic, antiviral (in-vitro and in-vivo), immunomodulatory activity and influence on mitotic divisions of three taxol derivatives: 10-deacetyl-baccatin III, methyl (N-benzoyl-(2'R,3'S)-3'-phenylisoserinate) and N-benzoyl-(2'R,3'S)-3'-phenylisoserine. | The Journal of pharmacy and pharmacology 20050601 |
| Characterization of an abeo-taxane: brevifoliol and derivatives. | Journal of natural products 20040501 |
| Effect of antiproliferative agents on vascular function in normal and in vitro balloon-injured porcine coronary arteries. | European journal of pharmacology 20031114 |
| Synthesis of biologically active taxol analogues with modified phenylisoserine side chains. | Journal of medicinal chemistry 19921030 |
Related Products
© 2019 Angene International Limited. All rights Reserved.