200,000+ products from a single source!
sales@angenechem.com
Home > Imidazoles > 132036-88-5
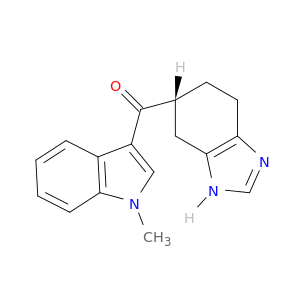
132036-88-5 | Methanone, (1-methyl-1H-indol-3-yl)[(6R)-4,5,6,7-tetrahydro-1H-benzimidazol-6-yl]-
CAS No: 132036-88-5 Catalog No: AG001037 MDL No:MFCD19686956
Product Description
Catalog Number:
AG001037
Chemical Name:
Methanone, (1-methyl-1H-indol-3-yl)[(6R)-4,5,6,7-tetrahydro-1H-benzimidazol-6-yl]-
CAS Number:
132036-88-5
Molecular Formula:
C17H17N3O
Molecular Weight:
279.3364
MDL Number:
MFCD19686956
IUPAC Name:
(1-methylindol-3-yl)-[(5R)-4,5,6,7-tetrahydro-3H-benzimidazol-5-yl]methanone
InChI:
InChI=1S/C17H17N3O/c1-20-9-13(12-4-2-3-5-16(12)20)17(21)11-6-7-14-15(8-11)19-10-18-14/h2-5,9-11H,6-8H2,1H3,(H,18,19)/t11-/m1/s1
InChI Key:
NTHPAPBPFQJABD-LLVKDONJSA-N
SMILES:
O=C(c1cn(c2c1cccc2)C)[C@@H]1CCc2c(C1)[nH]cn2
UNII:
7ZRO0SC54Y
Properties
Complexity:
413
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
279.137g/mol
Formal Charge:
0
Heavy Atom Count:
21
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
279.343g/mol
Monoisotopic Mass:
279.137g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
50.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.2
Literature
| Title | Journal |
|---|---|
| Population pharmacokinetics of ramosetron. | Journal of pharmacokinetics and pharmacodynamics 20160201 |
| A meta-analysis of prevention of postoperative nausea and vomiting: randomised controlled trials by Fujii et al. compared with other authors. | Anaesthesia 20121001 |
| Retraction record rocks community. | Nature 20120920 |
| Inhibitory effect of ramosetron on corticotropin releasing factor- and soybean oil-induced delays in gastric emptying in rats. | Journal of gastroenterology and hepatology 20120901 |
| Comparison of the antiemetic effect of ramosetron and combined ramosetron and midazolam in children: a double-blind, randomised clinical trial. | European journal of anaesthesiology 20120401 |
| Efficacy of ramosetron in the treatment of male patients with irritable bowel syndrome with diarrhea: a multicenter, randomized clinical trial, compared with mebeverine. | Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 20111201 |
| Treatment of IBS-D with 5-HT3 receptor antagonists vs spasmolytic agents: similar therapeutical effects from heterogeneous pharmacological targets. | Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 20111201 |
| Intravenous, oral, and the combination of intravenous and oral ramosetron for the prevention of nausea and vomiting after laparoscopic cholecystectomy: a randomized, double-blind, controlled trial. | Clinical therapeutics 20110901 |
| Retraction Notice: Current review of ramosetron in the prevention of postoperative nausea and vomiting. | Current drug safety 20110401 |
| Cisplatin-induced emesis: systematic review and meta-analysis of the ferret model and the effects of 5-HT₃ receptor antagonists. | Cancer chemotherapy and pharmacology 20110301 |
| Novel serotonin type 3 receptor partial agonists for the potential treatment of irritable bowel syndrome. | Bioorganic & medicinal chemistry letters 20110101 |
| Prevention of nausea and vomiting during termination of pregnancy. | International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics 20101001 |
| Prophylactic control of post-operative nausea and vomiting using ondansetron and ramosetron after cardiac surgery. | Acta anaesthesiologica Scandinavica 20100901 |
| Matching groups for studying postoperative nausea and vomiting: should we care? | Surgical endoscopy 20100701 |
| Comparison of ramosetron and ondansetron for control of post-operative nausea and vomiting following laparoscopic cholecystectomy. | Indian journal of medical sciences 20100601 |
| Comparison of the prophylactic anti-emetic efficacy of ramosetron and ondansetron in patients at high-risk for postoperative nausea and vomiting after total knee replacement. | Anaesthesia 20100501 |
| Ramosetron versus ondansetron for the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. | Surgical endoscopy 20100401 |
| A randomized, double-blind, parallel, comparative study to evaluate the efficacy and safety of ramosetron plus dexamethasone injection for the prevention of acute chemotherapy-induced nausea and vomiting. | Japanese journal of clinical oncology 20100401 |
| Serotonin 5-HT3 receptor antagonist for treatment of severe diabetic diarrhea. | Diabetes care 20100301 |
| Receptor occupancy theory-based analysis of interindividual differences in antiemetic effects of 5-HT3 receptor antagonists. | International journal of clinical oncology 20091201 |
| Comparison of ramosetron with ondansetron for prevention of postoperative nausea and vomiting in patients undergoing gynaecological surgery. | British journal of anaesthesia 20091001 |
| [Pharmacological and clinical profile of ramosetron hydrochloride (Irribow), a novel therapeutic agent for irritable bowel syndrome with diarrhea]. | Nihon yakurigaku zasshi. Folia pharmacologica Japonica 20090501 |
| Ramosetron for the prevention of nausea and vomiting during 5-fluorouracil-based chemoradiotherapy for pancreatico-biliary cancer. | Japanese journal of clinical oncology 20090201 |
| The effect of oral and IV ramosetron on postoperative nausea and vomiting in patients undergoing gynecological laparoscopy with total intravenous anesthesia. | Journal of anesthesia 20090101 |
| Preoperatively administered ramosetron oral disintegrating tablets for preventing nausea and vomiting associated with patient-controlled analgesia in breast cancer patients. | European journal of anaesthesiology 20080901 |
| Effect of ramosetron on patient-controlled analgesia related nausea and vomiting after spine surgery in highly susceptible patients: comparison with ondansetron. | Spine 20080801 |
| The effect of fluvoxamine on the pharmacokinetics, safety, and tolerability of ramosetron in healthy subjects. | European journal of clinical pharmacology 20080701 |
| Effects of serotonin 5-HT(3) receptor antagonists on CRF-induced abnormal colonic water transport and defecation in rats. | European journal of pharmacology 20080610 |
| The effect of paroxetine on the pharmacokinetics, safety, and tolerability of ramosetron in healthy subjects. | European journal of clinical pharmacology 20080601 |
| [Clinical evaluation of antiemetic effects of 5-hydroxytryptamine receptor type 3 (5HT3 receptor) antagonists based on changes in eating condition in cancer patients receiving chemotherapy]. | Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan 20080401 |
| Inhibitory effects of ramosetron, a potent and selective 5-HT3-receptor antagonist, on conditioned fear stress-induced abnormal defecation and normal defecation in rats: comparative studies with antidiarrheal and spasmolytic agents. | Journal of pharmacological sciences 20080201 |
| A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. | Scandinavian journal of gastroenterology 20080101 |
| A phase II trial of the novel serotonin type 3 receptor antagonist ramosetron in Japanese male and female patients with diarrhea-predominant irritable bowel syndrome. | Digestion 20080101 |
| Effect of ramosetron on conditioned emotional stress-induced colonic dysfunction as a model of irritable bowel syndrome in rats. | European journal of pharmacology 20071114 |
| Evaluation of the pharmacological profile of ramosetron, a novel therapeutic agent for irritable bowel syndrome. | Journal of pharmacological sciences 20070701 |
| [Comparison of antiemetic efficacy of 5-HT3 receptor antagonists in orthopedics cancer patients receiving high-dose chemotherapy]. | Gan to kagaku ryoho. Cancer & chemotherapy 20070301 |
| Pharmacological profile of ramosetron, a novel therapeutic agent for IBS. | Inflammopharmacology 20070201 |
| Ramosetron versus ondansetron in the prevention of chemotherapy-induced gastrointestinal side effects: A prospective randomized controlled study. | Chemotherapy 20070101 |
| Effects of serotonin-3 receptor antagonists on cytochrome P450 activities in human liver microsomes. | Biological & pharmaceutical bulletin 20060901 |
| [Comparison of ramosetron and azasetron for prevention of acute and delayed cisplatin-induced emesis in lung cancer patients]. | Gan to kagaku ryoho. Cancer & chemotherapy 20060501 |
| Comparison of ramosetron and granisetron for the prevention of acute and delayed emesis in Cisplatin-based chemotherapy: a randomized controlled trial. | Japanese journal of clinical oncology 20051201 |
| [A randomized crossover study of ramosetron plus dexamethasone for the prevention of nausea and vomiting induced by chemotherapy including cisplatin-comparison of ramosetron combined with 8 mg and 12 mg of dexamethasone]. | Gan to kagaku ryoho. Cancer & chemotherapy 20051201 |
| Exogenous melatonin delays gastric emptying rate in rats: role of CCK2 and 5-HT3 receptors. | Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 20051201 |
| Comparative clinical study of the anti-emetic effects of oral ramosetron and injected granisetron in patients with malignant glioma undergoing ACNU chemotherapy. | Neurologia medico-chirurgica 20050601 |
| [Effect of steroid on antiemetic for side effect of anticancer chemotherapy]. | Gan to kagaku ryoho. Cancer & chemotherapy 20050301 |
| A randomized clinical trial of a single dose of ramosetron for the prevention of vomiting after strabismus surgery in children: a dose-ranging study. | Archives of ophthalmology (Chicago, Ill. : 1960) 20050101 |
| Benefits and risks of granisetron versus ramosetron for nausea and vomiting after breast surgery: a randomized, double-blinded, placebo-controlled trial. | American journal of therapeutics 20040101 |
| A randomized, double-blind, placebo-controlled trial of ramosetron for preventing nausea and vomiting during termination of pregnancy. | International journal of obstetric anesthesia 20040101 |
| [Preventive effects of ramosetron and granisetron in prevention of gastrointestinal reaction associated with chemotherapeutic agents: a comparative study]. | Zhonghua yi xue za zhi 20031210 |
| A double-blind, crossover, randomized comparison of granisetron and ramosetron for the prevention of acute and delayed cisplatin-induced emesis in patients with gastrointestinal cancer: is patient preference a better primary endpoint? | Chemotherapy 20031201 |
| Randomized, double-blind, placebo-controlled, dosed-finding study of the antiemetic effects and tolerability of ramosetron in adults undergoing middle ear surgery. | Clinical therapeutics 20031201 |
| Results of a prospective, randomized, double-blind, placebo-controlled, dose-ranging trial to determine the effective dose of ramosetron for the prevention of vomiting after tonsillectomy in children. | Clinical therapeutics 20031201 |
| [Evaluation of QOL with orally disintegrating antiemetic tablets in outpatient chemotherapy]. | Gan to kagaku ryoho. Cancer & chemotherapy 20031001 |
| [Effects of Nasea on prevention of gastrointestinal side effects caused by chemotherapeutic drugs]. | Zhonghua yi xue za zhi 20030710 |
| Prophylactic antiemetic efficacy of granisetron or ramosetron in patients undergoing thyroidectomy. | Asian journal of surgery 20021001 |
| Clinical comparison of the selective serotonin3 antagonists ramosetron and granisetron in treating acute chemotherapy-induced emesis, nausea and anorexia. | Chinese medical sciences journal = Chung-kuo i hsueh k'o hsueh tsa chih 20020901 |
| The effect of the selective 5-HT(3) receptor agonist on ferret gut motility. | Life sciences 20020802 |
| Contribution of P-glycoprotein to efflux of ramosetron, a 5-HT3 receptor antagonist, across the blood-brain barrier. | The Journal of pharmacy and pharmacology 20020801 |
| Double-blind, placebo-controlled, dose-ranging study of ramosetron for the prevention of nausea and vomiting after thyroidectomy. | Clinical therapeutics 20020701 |
| Comparison of granisetron and ramosetron for the prevention of nausea and vomiting after thyroidectomy. | Clinical therapeutics 20020501 |
| Ramosetron, a 5-HT3 receptor antagonist for the control of nausea and vomiting. | Drugs of today (Barcelona, Spain : 1998) 20020201 |
| Effects of ondansetron, granisetron, ramosetron, and azasetron on human neutrophil functions. | Gynecologic and obstetric investigation 20020101 |
| Clinical evaluation of Ramosetron injections in the treatment of cisplatin-induced nausea and vomiting. | The Journal of international medical research 20020101 |
| Ramosetron for the prevention of cisplatin-induced acute emesis: a prospective randomized comparison with granisetron. | The Journal of international medical research 20020101 |
| Prophylaxis of nausea and vomiting after laparoscopic cholecystectomy with ramosetron: randomised controlled trial. | The European journal of surgery = Acta chirurgica 20020101 |
| A novel 5-HT3 receptor agonist, YM-31636, increases gastrointestinal motility without increasing abdominal pain. | European journal of pharmacology 20011109 |
| [Analysis of 5-HT3 receptor antagonist, ramosetron hydrochloride, based on receptor occupancy considering its active metabolite]. | Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan 20011101 |
| [Evaluation of efficacy of ramosetron orally disintegrating tablets and patient preference as to the dosage form in gynecological cancer chemotherapy]. | Gan to kagaku ryoho. Cancer & chemotherapy 20010801 |
| Ramosetron compared with granisetron for the prevention of vomiting following strabismus surgery in children. | The British journal of ophthalmology 20010601 |
| Ramosetron for the management of chemotherapy-induced gastrointestinal events in patients with hematological malignancies. | Methods and findings in experimental and clinical pharmacology 20010501 |
| Prevention of vomiting after tonsillectomy in children: granisetron versus ramosetron. | The Laryngoscope 20010201 |
| Ramosetron for preventing postoperative nausea and vomiting in women undergoing gynecological surgery. | Anesthesia and analgesia 20000201 |
| Effect of the 5-hydroxytryptamine3 (5-HT3)-receptor antagonist KB-R6933 on experimental diarrhea models. | Japanese journal of pharmacology 19990501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.