200,000+ products from a single source!
sales@angenechem.com
Home > Quinoline > 131802-60-3
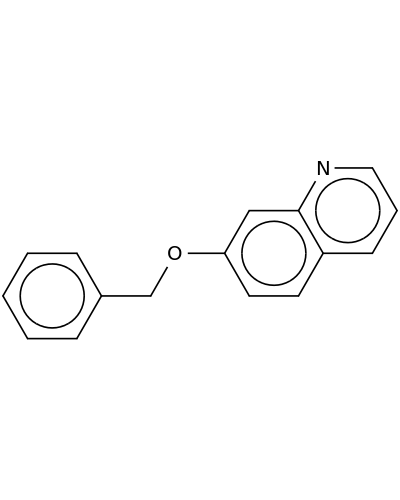
131802-60-3 | Quinoline, 7-(phenylmethoxy)-
CAS No: 131802-60-3 Catalog No: AG000ZRN MDL No:
Product Description
Catalog Number:
AG000ZRN
Chemical Name:
Quinoline, 7-(phenylmethoxy)-
CAS Number:
131802-60-3
Molecular Formula:
C16H13NO
Molecular Weight:
235.2805
IUPAC Name:
7-phenylmethoxyquinoline
InChI:
InChI=1S/C16H13NO/c1-2-5-13(6-3-1)12-18-15-9-8-14-7-4-10-17-16(14)11-15/h1-11H,12H2
InChI Key:
SIDLHXXVIBTSJZ-UHFFFAOYSA-N
SMILES:
c1ccc(cc1)COc1ccc2c(c1)nccc2
Properties
Complexity:
250
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
235.1g/mol
Formal Charge:
0
Heavy Atom Count:
18
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
235.286g/mol
Monoisotopic Mass:
235.1g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
22.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.9
Literature
| Title | Journal |
|---|---|
| Improvac does not modify the expression and activities of the major drug metabolizing enzymes cytochrome P450 3A and 2C in pigs. | Vaccine 20120521 |
| Comparison of three fluorescent CYP3A substrates in two vertebrate models: pig and Atlantic salmon. | Animal : an international journal of animal bioscience 20120401 |
| Exploration of the amine terminus in a novel series of 1,2,4-triazolo-3-yl-azabicyclo[3.1.0]hexanes as selective dopamine D3 receptor antagonists. | Journal of medicinal chemistry 20101014 |
| Computational approach to characterization of human liver drug-metabolizing enzymes. | European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences 20101009 |
| Assessment of cytochrome P450 fluorometric substrates with rainbow trout and killifish exposed to dexamethasone, pregnenolone-16alpha-carbonitrile, rifampicin, and beta-naphthoflavone. | Aquatic toxicology (Amsterdam, Netherlands) 20100510 |
| Effects of cytochrome P450 inhibitors on the biotransformation of fluorogenic substrates by adult male rat liver microsomes and cDNA-expressed rat cytochrome P450 isoforms. | Toxicological sciences : an official journal of the Society of Toxicology 20100201 |
| 1,2,4-Triazolyl azabicyclo[3.1.0]hexanes: a new series of potent and selective dopamine D(3) receptor antagonists. | Journal of medicinal chemistry 20100114 |
| DP7, a novel dihydropyridine multidrug resistance reverter, shows only weak inhibitory activity on human CYP3A enzyme(s). | European journal of pharmacology 20090701 |
| Ligand diversity of human and chimpanzee CYP3A4: activation of human CYP3A4 by lithocholic acid results from positive selection. | Drug metabolism and disposition: the biological fate of chemicals 20090601 |
| In vitro CYP3A4 metabolism: inhibition by Echinacea purpurea and choice of substrate for the evaluation of herbal inhibition. | Basic & clinical pharmacology & toxicology 20081101 |
| Multiple substrate binding by cytochrome P450 3A4: estimation of the number of bound substrate molecules. | Drug metabolism and disposition: the biological fate of chemicals 20081001 |
| Effect of glutathione on homo- and heterotropic cooperativity in cytochrome P450 3A4. | Archives of biochemistry and biophysics 20080315 |
| Structure-activity relationship and substrate-dependent phenomena in effects of ginsenosides on activities of drug-metabolizing P450 enzymes. | PloS one 20080101 |
| Time-dependent inactivation of P450 3A4 by raloxifene: identification of Cys239 as the site of apoprotein alkylation. | Chemical research in toxicology 20070601 |
| Engineering of cytochrome P450 3A4 for enhanced peroxide-mediated substrate oxidation using directed evolution and site-directed mutagenesis. | Drug metabolism and disposition: the biological fate of chemicals 20061201 |
| Time-resolved fluorescence studies of heterotropic ligand binding to cytochrome P450 3A4. | Biochemistry 20061010 |
| CYP3A4 activity in four different animal species liver microsomes using 7-benzyloxyquinoline and HPLC/spectrofluorometric determination. | Journal of pharmaceutical and biomedical analysis 20060123 |
| Cytochrome P450 3A expression and activity in the rabbit lacrimal gland: glucocorticoid modulation and the impact on androgen metabolism. | Investigative ophthalmology & visual science 20051201 |
| Role of cytochrome B5 in modulating peroxide-supported cyp3a4 activity: evidence for a conformational transition and cytochrome P450 heterogeneity. | Drug metabolism and disposition: the biological fate of chemicals 20050801 |
| Cytochrome P450 3A4-catalyzed testosterone 6beta-hydroxylation stereochemistry, kinetic deuterium isotope effects, and rate-limiting steps. | The Journal of biological chemistry 20050520 |
| Suppression of drug-metabolizing enzymes and efflux transporters in the intestine of endotoxin-treated rats. | Drug metabolism and disposition: the biological fate of chemicals 20040101 |
| High-throughput screening for the assessment of time-dependent inhibitions of new drug candidates on recombinant CYP2D6 and CYP3A4 using a single concentration method. | Xenobiotica; the fate of foreign compounds in biological systems 20040101 |
| Homotropic versus heterotopic cooperativity of cytochrome P450eryF: a substrate oxidation and spectral titration study. | Drug metabolism and disposition: the biological fate of chemicals 20030401 |
| Cytochrome P450 fluorometric substrates: identification of isoform-selective probes for rat CYP2D2 and human CYP3A4. | Drug metabolism and disposition: the biological fate of chemicals 20020701 |
| 7-Benzyloxyquinoline oxidation by P450eryF A245T: finding of a new fluorescent substrate probe. | Chemical research in toxicology 20020601 |
| Site-directed mutagenesis of cytochrome P450eryF: implications for substrate oxidation, cooperativity, and topology of the active site. | Chemical research in toxicology 20020601 |
| Evaluation of 7-benzyloxy-4-trifluoromethylcoumarin, some other 7-hydroxy-4-trifluoromethylcoumarin derivatives and 7-benzyloxyquinoline as fluorescent substrates for rat hepatic cytochrome P450 enzymes. | Xenobiotica; the fate of foreign compounds in biological systems 20011201 |
| Testosterone, 7-benzyloxyquinoline, and 7-benzyloxy-4-trifluoromethyl-coumarin bind to different domains within the active site of cytochrome P450 3A4. | Drug metabolism and disposition: the biological fate of chemicals 20011101 |
| Metabolism of 2,5-bis(trifluoromethyl)-7-benzyloxy-4-trifluoromethylcoumarin by human hepatic CYP isoforms: evidence for selectivity towards CYP3A4. | Xenobiotica; the fate of foreign compounds in biological systems 20010401 |
Related Products
© 2019 Angene International Limited. All rights Reserved.