200,000+ products from a single source!
sales@angenechem.com
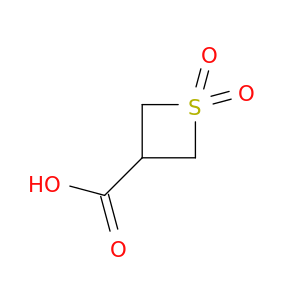
Home > Other Building Blocks > 13159-28-9
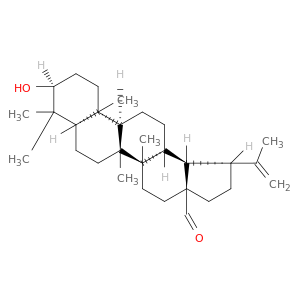
13159-28-9 | Lup-20(29)-en-28-al, 3-hydroxy-, (3β)-
CAS No: 13159-28-9 Catalog No: AG000ZGY MDL No:MFCD12407180
Product Description
Catalog Number:
AG000ZGY
Chemical Name:
Lup-20(29)-en-28-al, 3-hydroxy-, (3β)-
CAS Number:
13159-28-9
Molecular Formula:
C30H48O2
Molecular Weight:
440.7009
MDL Number:
MFCD12407180
IUPAC Name:
(1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-hydroxy-5a,5b,8,8,11a-pentamethyl-1-prop-1-en-2-yl-1,2,3,4,5,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydrocyclopenta[a]chrysene-3a-carbaldehyde
InChI:
InChI=1S/C30H48O2/c1-19(2)20-10-15-30(18-31)17-16-28(6)21(25(20)30)8-9-23-27(5)13-12-24(32)26(3,4)22(27)11-14-29(23,28)7/h18,20-25,32H,1,8-17H2,2-7H3/t20-,21+,22-,23+,24-,25+,27-,28+,29+,30+/m0/s1
InChI Key:
FELCJAPFJOPHSD-ROUWMTJPSA-N
SMILES:
O=C[C@@]12CC[C@H]([C@@H]2[C@@H]2[C@](CC1)(C)[C@]1(C)CC[C@@H]3[C@]([C@H]1CC2)(C)CC[C@@H](C3(C)C)O)C(=C)C
Properties
Complexity:
814
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
10
Defined Bond Stereocenter Count:
0
Exact Mass:
440.365g/mol
Formal Charge:
0
Heavy Atom Count:
32
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
440.712g/mol
Monoisotopic Mass:
440.365g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
37.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
8.1
Literature
| Title | Journal |
|---|---|
| CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. | Plant & cell physiology 20111201 |
| Antifungal metabolites from the roots of Diospyros virginiana by overpressure layer chromatography. | Chemistry & biodiversity 20111201 |
| Triterpene derivatives that inhibit human immunodeficiency virus type 1 replication. | Bioorganic & medicinal chemistry letters 20110101 |
| Phenylpropanoids from the stem bark of Jacaranda mimosaefolia. | Natural product research 20110101 |
| Synergistic antimicrobial activity between pentacyclic triterpenoids and antibiotics against Staphylococcus aureus strains. | Annals of clinical microbiology and antimicrobials 20110101 |
| An anti-influenza component of the bark of Alnus japonica. | Archives of pharmacal research 20100301 |
| Synthesis and anti-leishmanial activity of heterocyclic betulin derivatives. | Bioorganic & medicinal chemistry 20100215 |
| C-Glucoside xanthone from the stem bark extract of Bersama engleriana. | Pharmacognosy research 20100101 |
| Betulin-derived compounds as inhibitors of alphavirus replication. | Journal of natural products 20091101 |
| Analysis of pentacyclic triterpenes by LC-MS. A comparative study between APCI and APPI. | Journal of mass spectrometry : JMS 20090101 |
| alpha-Glucosidase inhibitory activity of triterpenoids from Cichorium intybus. | Journal of natural products 20080501 |
| Lanostane-type triterpenoids from Diospyros discolor. | Chemical & pharmaceutical bulletin 20070601 |
| Two new flavanone glycosides of Jasminum lanceolarium and their anti-oxidant activities. | Chemical & pharmaceutical bulletin 20070301 |
| Ceanothane- and lupane-type triterpenes with antiplasmodial and antimycobacterial activities from Ziziphus cambodiana. | Chemical & pharmaceutical bulletin 20060401 |
| Erythrocyte membrane modifying agents and the inhibition of Plasmodium falciparum growth: structure-activity relationships for betulinic acid analogues. | Bioorganic & medicinal chemistry 20040102 |
| Differentiation- and apoptosis-inducing activities by pentacyclic triterpenes on a mouse melanoma cell line. | Journal of natural products 20020501 |
| Cytotoxic activity of moronic acid and identification of the new triterpene 3,4-seco-olean-18-ene-3,28-dioic acid from Phoradendron reichenbachianum. | Planta medica 20010701 |
| Inhibitory effects of triterpenoids and sterols on human immunodeficiency virus-1 reverse transcriptase. | Lipids 20010501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.