200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 131021-99-3
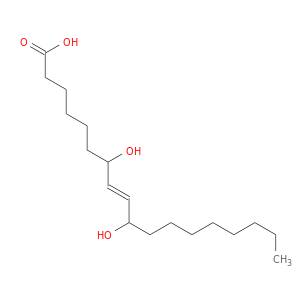
131021-99-3 | 8-Octadecenoic acid, 7,10-dihydroxy-, (8E)-
CAS No: 131021-99-3 Catalog No: AG000VC7 MDL No:
Product Description
Catalog Number:
AG000VC7
Chemical Name:
8-Octadecenoic acid, 7,10-dihydroxy-, (8E)-
CAS Number:
131021-99-3
Molecular Formula:
C18H34O4
Molecular Weight:
314.4602
IUPAC Name:
7,10-dihydroxyoctadec-8-enoic acid
InChI:
InChI=1S/C18H34O4/c1-2-3-4-5-6-8-11-16(19)14-15-17(20)12-9-7-10-13-18(21)22/h14-17,19-20H,2-13H2,1H3,(H,21,22)
InChI Key:
IPRLELFMTNDNMN-UHFFFAOYSA-N
SMILES:
CCCCCCCCC(/C=C/C(CCCCCC(=O)O)O)O
Properties
Complexity:
289
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
314.246g/mol
Formal Charge:
0
Heavy Atom Count:
22
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
314.466g/mol
Monoisotopic Mass:
314.246g/mol
Rotatable Bond Count:
15
Topological Polar Surface Area:
77.8A^2
Undefined Atom Stereocenter Count:
2
Undefined Bond Stereocenter Count:
1
XLogP3:
4.3
Literature
| Title | Journal |
|---|---|
| One-step production of a biologically active novel furan fatty acid from 7,10-dihydroxy-8(E)-octadecenoic acid. | Journal of agricultural and food chemistry 20110810 |
| Production of 7,10-dihydroxy-8(E)-octadecenoic acid from olive oil by Pseudomonas aeruginosa PR3. | Applied microbiology and biotechnology 20110301 |
| alpha-Glucosidase inhibitory activities of 10-hydroxy-8(E)-octadecenoic acid: an intermediate of bioconversion of oleic acid to 7,10-dihydroxy-8(E)-octadecenoic acid. | New biotechnology 20100930 |
| Identification of oxylipins with antifungal activity by LC-MS/MS from the supernatant of Pseudomonas 42A2. | Chemistry and physics of lipids 20100501 |
| Liquid chromatography/tandem mass spectrometric analysis of 7,10-dihydroxyoctadecenoic acid, its isotopomers, and other 7,10-dihydroxy fatty acids formed by Pseudomonas aeruginosa 42A2. | Rapid communications in mass spectrometry : RCM 20100301 |
| Lipase-catalyzed production of a bioactive fatty amide derivative of 7,10-dihydroxy-8(E)-octadecenoic acid. | Bioresource technology 20090201 |
| Production of 10-hydroxy-8(E)-octadecenoic acid from oleic acid conversion by strains of Pseudomonas aeruginosa. | Current microbiology 20081101 |
| Characterization of fatty amides produced by lipase-catalyzed amidation of multi-hydroxylated fatty acids. | Bioresource technology 20080501 |
| Environmental optimization for bioconversion of triolein into 7,10-dihydroxy-8(E)-octadecenoic acid by Pseudomonas aeruginosa PR3. | Applied microbiology and biotechnology 20080301 |
| New bioactive fatty acids. | Asia Pacific journal of clinical nutrition 20080101 |
| Production and identification of a novel compound, 7,10-dihydroxy-8(E)-hexadecenoic acid from palmitoleic acid by Pseudomonas aeruginosa PR3. | Applied microbiology and biotechnology 20070501 |
| Production of 7, 10-dihydroxy-8(E)-octadecenoic acid from triolein via lipase induction by Pseudomonas aeruginosa PR3. | Applied microbiology and biotechnology 20070201 |
| Diversity of oleic acid, ricinoleic acid and linoleic acid conversions among Pseudomonas aeruginosa strains. | Current microbiology 20041001 |
| Isolation and characterization of a lipoxygenase from Pseudomonas 42A2 responsible for the biotransformation of oleic acid into ( S )-( E )-10-hydroxy-8-octadecenoic acid. | Antonie van Leeuwenhoek 20040201 |
| Factors influencing the production of a novel compound, 7,10-dihydroxy-8(E)-octadecenoic acid, by Pseudomonas aeruginosa PR3 (NRRL B-18602) in batch cultures. | Current microbiology 20030901 |
| A facile reactor process for producing 7,10-dihydroxy-8(E)-octadecenoic acid from oleic acid conversion by Pseudomonas aeruginosa. | Biotechnology letters 20030101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.