200,000+ products from a single source!
sales@angenechem.com
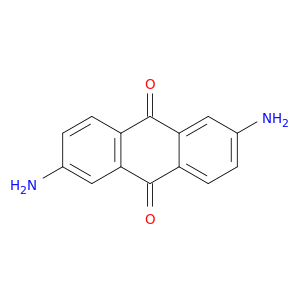
131-14-6 | 9,10-Anthracenedione, 2,6-diamino-
CAS No: 131-14-6 Catalog No: AG000VAI MDL No:MFCD00001234
Product Description
Catalog Number:
AG000VAI
Chemical Name:
9,10-Anthracenedione, 2,6-diamino-
CAS Number:
131-14-6
Molecular Formula:
C14H10N2O2
Molecular Weight:
238.2414
MDL Number:
MFCD00001234
IUPAC Name:
2,6-diaminoanthracene-9,10-dione
InChI:
InChI=1S/C14H10N2O2/c15-7-1-3-9-11(5-7)14(18)10-4-2-8(16)6-12(10)13(9)17/h1-6H,15-16H2
InChI Key:
WQOWBWVMZPPPGX-UHFFFAOYSA-N
SMILES:
Nc1ccc2c(c1)C(=O)c1c(C2=O)cc(cc1)N
EC Number:
205-013-7
UNII:
3DY0EUZ2ZM
NSC Number:
39935
Properties
Complexity:
346
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
238.074g/mol
Formal Charge:
0
Heavy Atom Count:
18
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
238.246g/mol
Monoisotopic Mass:
238.074g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
86.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.5
Literature
| Title | Journal |
|---|---|
| Determination of the optical GAP in thin films of amorphous dilithium phthalocyanine using the Tauc and Cody models. | Molecules (Basel, Switzerland) 20120824 |
| Study of preferential solvation of 2,6-diaminoanthraquinone in binary mixtures by absorption and fluorescence studies. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20080801 |
| Solvent polarity induced structural changes in 2,6-diamino-9,10-anthraquinone dye. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20080101 |
| Photophysical behaviour of 2,6-diaminoanthraquinone in different solvents and at various pH. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20071101 |
| Long-wavelength analogue of PRODAN: synthesis and properties of Anthradan, a fluorophore with a 2,6-donor-acceptor anthracene structure. | The Journal of organic chemistry 20061222 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| A homogeneous time-resolved fluorescence detection of telomerase activity. | Analytical biochemistry 20041001 |
| Nanowells on silica particles in water containing long-distance porphyrin heterodimers. | Journal of the American Chemical Society 20030903 |
| Electronic absorption spectra of amino substituted anthraquinones and their interpretation using the ZINDO/S and AM1 methods. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20030501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.