200,000+ products from a single source!
sales@angenechem.com
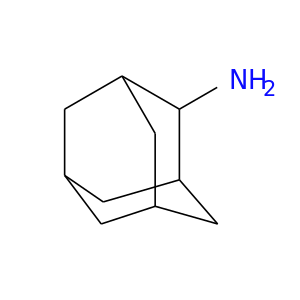
13074-39-0 | Tricyclo[3.3.1.13,7]decan-2-amine
CAS No: 13074-39-0 Catalog No: AG000UW6 MDL No:MFCD00078225
Product Description
Catalog Number:
AG000UW6
Chemical Name:
Tricyclo[3.3.1.13,7]decan-2-amine
CAS Number:
13074-39-0
Molecular Formula:
C10H17N
Molecular Weight:
151.2487
MDL Number:
MFCD00078225
IUPAC Name:
adamantan-2-amine
InChI:
InChI=1S/C10H17N/c11-10-8-2-6-1-7(4-8)5-9(10)3-6/h6-10H,1-5,11H2
InChI Key:
QZWNXXINFABALM-UHFFFAOYSA-N
SMILES:
NC1C2CC3CC1CC(C2)C3
NSC Number:
127842
Properties
Complexity:
148
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
151.136g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
151.253g/mol
Monoisotopic Mass:
151.136g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
26A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.7
Literature
| Title | Journal |
|---|---|
| Aminoadamantanes containing monoterpene-derived fragments as potent tyrosyl-DNA phosphodiesterase 1 inhibitors. | Bioorganic chemistry 20180201 |
| Potentiation of azole antifungals by 2-adamantanamine. | Antimicrobial agents and chemotherapy 20130801 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Magic angle spinning NMR investigation of influenza A M2(18-60): support for an allosteric mechanism of inhibition. | Journal of the American Chemical Society 20100818 |
| Interaction of aminoadamantane derivatives with the influenza A virus M2 channel-docking using a pore blocking model. | Bioorganic & medicinal chemistry letters 20100715 |
| The prediction of (1)H chemical shifts in amines: a semiempirical and ab initio investigation. | Magnetic resonance in chemistry : MRC 20070901 |
| [Cytosine demethylation in the tyrosine hydroxylase gene promoter in the hypothalamus cells of the rat brain under the action of an aminoadamantane derivative Ladasten]. | Genetika 20060701 |
| Simultaneous liquid chromatographic assay of amantadine and its four related compounds in phosphate-buffered saline using 4-fluoro-7-nitro-2,1,3-benzoxadiazole as a fluorescent derivatization reagent. | Biomedical chromatography : BMC 20060501 |
| Simultaneous determination of the binding of amantadine and its analogues to synthetic melanin by liquid chromatography after precolumn derivatization with dansyl chloride. | Journal of chromatographic science 20050401 |
| [DNA macroarray analysis of gene expression changes in rat brain after single administration of 2-aminoadamantane compound]. | Molekuliarnaia biologiia 20050101 |
| Liquid chromatographic determination of 1-adamantanamine and 2-adamantanamine in human plasma after pre-column derivatization with o-phthalaldehyde and 1-thio-beta-D-glucose. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20040125 |
| Synthesis and antimicrobial activity evaluation of some new adamantane derivatives. | Arzneimittel-Forschung 20020101 |
| [Immunotropic activity of a potential antiparkinson agent himantane]. | Eksperimental'naia i klinicheskaia farmakologiia 20010101 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.