200,000+ products from a single source!
sales@angenechem.com
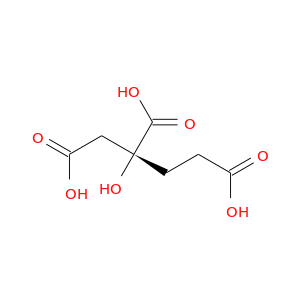
13052-73-8 | 1,2,4-Butanetricarboxylic acid, 2-hydroxy-, (2R)-
CAS No: 13052-73-8 Catalog No: AG000UK0 MDL No:
Product Description
Catalog Number:
AG000UK0
Chemical Name:
1,2,4-Butanetricarboxylic acid, 2-hydroxy-, (2R)-
CAS Number:
13052-73-8
Molecular Formula:
C7H10O7
Molecular Weight:
206.1501
IUPAC Name:
(2R)-2-hydroxybutane-1,2,4-tricarboxylic acid
InChI:
InChI=1S/C7H10O7/c8-4(9)1-2-7(14,6(12)13)3-5(10)11/h14H,1-3H2,(H,8,9)(H,10,11)(H,12,13)/t7-/m1/s1
InChI Key:
XKJVEVRQMLKSMO-SSDOTTSWSA-N
SMILES:
OC(=O)CC[C@](C(=O)O)(CC(=O)O)O
Properties
Complexity:
259
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
206.043g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
7
Hydrogen Bond Donor Count:
4
Isotope Atom Count:
0
Molecular Weight:
206.15g/mol
Monoisotopic Mass:
206.043g/mol
Rotatable Bond Count:
6
Topological Polar Surface Area:
132A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-1.7
Literature
| Title | Journal |
|---|---|
| Electronic dimensions of FeMo-co, the active site of nitrogenase, and its catalytic intermediates. | Inorganic chemistry 20110103 |
| Formation of a homocitrate-free iron-molybdenum cluster on NifEN: implications for the role of homocitrate in nitrogenase assembly. | Dalton transactions (Cambridge, England : 2003) 20100328 |
| Mechanism of substrate recognition and insight into feedback inhibition of homocitrate synthase from Thermus thermophilus. | The Journal of biological chemistry 20100205 |
| Transcriptional upregulation of four genes of the lysine biosynthetic pathway by homocitrate accumulation in Penicillium chrysogenum: homocitrate as a sensor of lysine-pathway distress. | Microbiology (Reading, England) 20091201 |
| Host plant genome overcomes the lack of a bacterial gene for symbiotic nitrogen fixation. | Nature 20091126 |
| Formations of mixed-valence oxovanadiumV,IV citrates and homocitrate with N-heterocycle chelated ligand. | Inorganic chemistry 20081006 |
| Evidence for nifU and nifS participation in the biosynthesis of the iron-molybdenum cofactor of nitrogenase. | The Journal of biological chemistry 20071221 |
| Acid-base chemical mechanism of homocitrate synthase from Saccharomyces cerevisiae. | Biochemistry 20061003 |
| Syntheses, spectroscopies and structures of molybdenum(VI) complexes with homocitrate. | Inorganic chemistry 20061002 |
| Evidence for a dynamic role for homocitrate during nitrogen fixation: the effect of substitution at the alpha-Lys426 position in MoFe-protein of Azotobacter vinelandii. | The Biochemical journal 20060715 |
| Kinetics and product analysis of the reaction catalysed by recombinant homoaconitase from Thermus thermophilus. | The Biochemical journal 20060615 |
| Synthesis of trimethyl (2S,3R)- and (2R,3R)-[2-2H1]-homocitrates and dimethyl (2S,3R)- and (2R,3R)-[2-2H1]-homocitrate lactones-an assay for the stereochemical outcome of the reaction catalysed both by homocitrate synthase and by the Nif-V protein. | Organic & biomolecular chemistry 20060207 |
| Binding modes for the first coupled electron and proton addition to FeMoco of nitrogenase. | Journal of the American Chemical Society 20020501 |
| Mechanistic features and structure of the nitrogenase alpha-Gln195 MoFe protein. | Biochemistry 20010213 |
| Requirement of homocitrate for the transfer of a 49V-labeled precursor of the iron-vanadium cofactor from VnfX to nif-apodinitrogenase. | The Journal of biological chemistry 20010209 |
Related Products
© 2019 Angene International Limited. All rights Reserved.