200,000+ products from a single source!
sales@angenechem.com
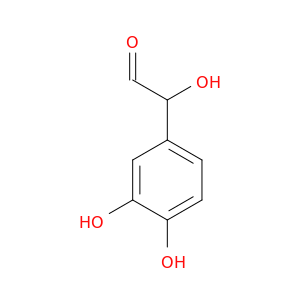
13023-73-9 | Benzeneacetaldehyde, α,3,4-trihydroxy-
CAS No: 13023-73-9 Catalog No: AG000U72 MDL No:
Product Description
Catalog Number:
AG000U72
Chemical Name:
Benzeneacetaldehyde, α,3,4-trihydroxy-
CAS Number:
13023-73-9
Molecular Formula:
C8H8O4
Molecular Weight:
168.1467
IUPAC Name:
2-(3,4-dihydroxyphenyl)-2-hydroxyacetaldehyde
InChI:
InChI=1S/C8H8O4/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-4,8,10-12H
InChI Key:
YUGMCLJIWGEKCK-UHFFFAOYSA-N
SMILES:
O=CC(c1ccc(c(c1)O)O)O
Properties
Complexity:
159
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
168.042g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
168.148g/mol
Monoisotopic Mass:
168.042g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
77.8A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.6
Literature
| Title | Journal |
|---|---|
| Neurotoxicity and metabolism of the catecholamine-derived 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. | Pharmacological reviews 20070601 |
| Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. | Neurotoxicology 20040101 |
| Convenient syntheses of biogenic aldehydes, 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde. | Bioorganic chemistry 20030401 |
| 3,4-Dihydroxyphenylacetaldehyde and hydrogen peroxide generate a hydroxyl radical: possible role in Parkinson's disease pathogenesis. | Brain research. Molecular brain research 20010910 |
| Catecholamine monoamine oxidase a metabolite in adrenergic neurons is cytotoxic in vivo. | Brain research 20010209 |
Related Products
© 2019 Angene International Limited. All rights Reserved.