200,000+ products from a single source!
sales@angenechem.com
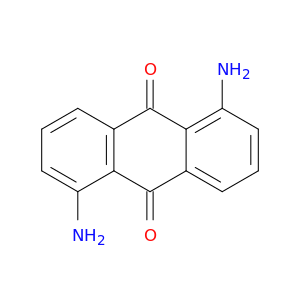
129-44-2 | 9,10-Anthracenedione, 1,5-diamino-
CAS No: 129-44-2 Catalog No: AG000YRG MDL No:MFCD00001226
Product Description
Catalog Number:
AG000YRG
Chemical Name:
9,10-Anthracenedione, 1,5-diamino-
CAS Number:
129-44-2
Molecular Formula:
C14H10N2O2
Molecular Weight:
238.2414
MDL Number:
MFCD00001226
IUPAC Name:
1,5-diaminoanthracene-9,10-dione
InChI:
InChI=1S/C14H10N2O2/c15-9-5-1-3-7-11(9)14(18)8-4-2-6-10(16)12(8)13(7)17/h1-6H,15-16H2
InChI Key:
VWBVCOPVKXNMMZ-UHFFFAOYSA-N
SMILES:
Nc1cccc2c1C(=O)c1cccc(c1C2=O)N
EC Number:
204-947-2
UNII:
3ZXX3HK358
Properties
Complexity:
346
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
238.074g/mol
Formal Charge:
0
Heavy Atom Count:
18
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
238.246g/mol
Monoisotopic Mass:
238.074g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
86.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.6
Literature
| Title | Journal |
|---|---|
| 1,5-Diamino-2,6-dibromo-9,10-anthraquinone. | Acta crystallographica. Section E, Structure reports online 20120301 |
| Construction of an organic crystal structural model based on combined electron and powder X-ray diffraction data and the charge flipping algorithm. | Ultramicroscopy 20110601 |
| Molecular recognition of 1,5 diamino anthraquinone by p-tert-butyl-calix(8)arene. | Journal of fluorescence 20100901 |
| Estrogenic activity of anthraquinone derivatives: in vitro and in silico studies. | Chemical research in toxicology 20100816 |
| Patterned growth of vertically aligned organic nanowire waveguide arrays. | ACS nano 20100323 |
| Preferential solvation studies of 1, 5-diaminoanthraquinone in binary liquid mixtures. | Journal of fluorescence 20100101 |
| Estimation of first excited singlet-state dipole moments of aminoanthraquinones by solvatochromic method. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20090401 |
| Vertical organic nanowire arrays: controlled synthesis and chemical sensors. | Journal of the American Chemical Society 20090311 |
| Valence-state analysis through spectroelectrochemistry in a series of quinonoid-bridged diruthenium complexes [(acac)(2)Ru(mu-L)Ru(acac)(2)](n) (n=+2, +1, 0, -1, -2). | Chemistry (Weinheim an der Bergstrasse, Germany) 20080101 |
| Productive synthesis and properties of polydiaminoanthraquinone and its pure self-stabilized nanoparticles with widely adjustable electroconductivity. | Chemistry (Weinheim an der Bergstrasse, Germany) 20070101 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Electronic absorption spectra of amino substituted anthraquinones and their interpretation using the ZINDO/S and AM1 methods. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20030501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.