200,000+ products from a single source!
sales@angenechem.com
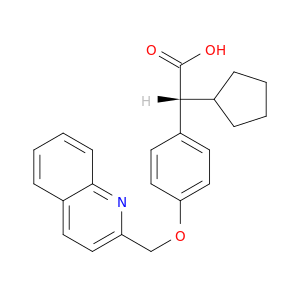
128253-31-6 | Benzeneacetic acid, α-cyclopentyl-4-(2-quinolinylmethoxy)-, (αR)-
CAS No: 128253-31-6 Catalog No: AG000XWK MDL No:
Product Description
Catalog Number:
AG000XWK
Chemical Name:
Benzeneacetic acid, α-cyclopentyl-4-(2-quinolinylmethoxy)-, (αR)-
CAS Number:
128253-31-6
Molecular Formula:
C23H23NO3
Molecular Weight:
361.4336
IUPAC Name:
(2R)-2-cyclopentyl-2-[4-(quinolin-2-ylmethoxy)phenyl]acetic acid
InChI:
InChI=1S/C23H23NO3/c25-23(26)22(17-6-1-2-7-17)18-10-13-20(14-11-18)27-15-19-12-9-16-5-3-4-8-21(16)24-19/h3-5,8-14,17,22H,1-2,6-7,15H2,(H,25,26)/t22-/m1/s1
InChI Key:
ZEYYDOLCHFETHQ-JOCHJYFZSA-N
SMILES:
OC(=O)[C@@H](c1ccc(cc1)OCc1ccc2c(n1)cccc2)C1CCCC1
UNII:
JXH6X663L0
Properties
Complexity:
482
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
361.168g/mol
Formal Charge:
0
Heavy Atom Count:
27
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
361.441g/mol
Monoisotopic Mass:
361.168g/mol
Rotatable Bond Count:
6
Topological Polar Surface Area:
59.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
5.4
Literature
| Title | Journal |
|---|---|
| Identification of potent Yes1 kinase inhibitors using a library screening approach. | Bioorganic & medicinal chemistry letters 20130801 |
| 5-Lipoxygenase-activating protein (FLAP) inhibitors. Part 4: development of 3-[3-tert-butylsulfanyl-1-[4-(6-ethoxypyridin-3-yl)benzyl]-5-(5-methylpyridin-2-ylmethoxy)-1H-indol-2-yl]-2,2-dimethylpropionic acid (AM803), a potent, oral, once daily FLAP inhibitor. | Journal of medicinal chemistry 20111208 |
| Inhibition of 5-lipoxygenase-activating protein abrogates experimental liver injury: role of Kupffer cells. | Journal of leukocyte biology 20051001 |
| Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trial. | JAMA 20050511 |
| Licofelone, an inhibitor of cyclooxygenase and 5-lipoxygenase, specifically inhibits cyclooxygenase-1-dependent platelet activation. | European journal of pharmacology 20040319 |
| Interactions among three classes of mediators explain antigen-induced bronchoconstriction in the isolated perfused and ventilated guinea pig lung. | The Journal of pharmacology and experimental therapeutics 20031001 |
| Inhibition of 5-lipoxygenase induces cell growth arrest and apoptosis in rat Kupffer cells: implications for liver fibrosis. | FASEB journal : official publication of the Federation of American Societies for Experimental Biology 20030901 |
| Evidence that 5-lipoxygenase and acetylated cyclooxygenase 2-derived eicosanoids regulate leukocyte-endothelial adherence in response to aspirin. | British journal of pharmacology 20030801 |
| Inhibition of 5-lipoxygenase activating protein decreases proteinuria in diabetic rats. | Journal of nephrology 20030101 |
| A randomized, placebo-controlled trial of a leukotriene synthesis inhibitor in patients with COPD. | Chest 20020701 |
| Study of the role of leukotriene B()4 in abnormal function of human subchondral osteoarthritis osteoblasts: effects of cyclooxygenase and/or 5-lipoxygenase inhibition. | Arthritis and rheumatism 20020701 |
| Synthesis of interleukin 1beta, tumor necrosis factor-alpha, and interstitial collagenase (MMP-1) is eicosanoid dependent in human osteoarthritis synovial membrane explants: interactions with antiinflammatory cytokines. | The Journal of rheumatology 20020301 |
| Inhibition of 5-LO activating protein (FLAP) activity decreases proteinuria in streptozotocin (STZ)-induced diabetic rats. | Advances in experimental medicine and biology 20020101 |
| MUC5AC mucin release from human airways in vitro: effects of indomethacin and Bay X1005. | Mediators of inflammation 20010201 |
| Leukotriene C4 is a tight-binding inhibitor of microsomal glutathione transferase-1. Effects of leukotriene pathway modifiers. | The Journal of biological chemistry 19990122 |
| Pharmacological modulation of human platelet leukotriene C4-synthase. | Biochemical pharmacology 19970321 |
| Mode of action of the new selective leukotriene synthesis inhibitor BAY X 1005 ((R)-2-[4-(quinolin-2-yl-methoxy)phenyl]-2-cyclopentyl acetic acid) and structurally related compounds. | Biochemical pharmacology 19930107 |
| Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. | Nature 19900118 |
Related Products
© 2019 Angene International Limited. All rights Reserved.