200,000+ products from a single source!
sales@angenechem.com
Home > Other Heterocycles > 127414-85-1
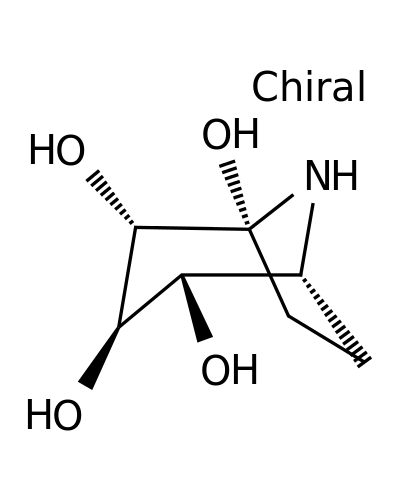
127414-85-1 | 8-Azabicyclo[3.2.1]octane-1,2,3,4-tetrol, (1R,2S,3R,4S,5R)-
CAS No: 127414-85-1 Catalog No: AG000X1R MDL No:MFCD14702344
Product Description
Catalog Number:
AG000X1R
Chemical Name:
8-Azabicyclo[3.2.1]octane-1,2,3,4-tetrol, (1R,2S,3R,4S,5R)-
CAS Number:
127414-85-1
Molecular Formula:
C7H13NO4
Molecular Weight:
175.1824
MDL Number:
MFCD14702344
IUPAC Name:
(1R,2S,3R,4S,5R)-8-azabicyclo[3.2.1]octane-1,2,3,4-tetrol
InChI:
InChI=1S/C7H13NO4/c9-4-3-1-2-7(12,8-3)6(11)5(4)10/h3-6,8-12H,1-2H2/t3-,4+,5-,6+,7-/m1/s1
InChI Key:
FXFBVZOJVHCEDO-IBISWUOJSA-N
SMILES:
O[C@H]1[C@H]2CC[C@](N2)([C@H]([C@@H]1O)O)O
Properties
Complexity:
200
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
5
Defined Bond Stereocenter Count:
0
Exact Mass:
175.084g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
5
Isotope Atom Count:
0
Molecular Weight:
175.184g/mol
Monoisotopic Mass:
175.084g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
93A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-2.4
Literature
| Title | Journal |
|---|---|
| Total synthesis of ent-calystegine B4 via nitro-Michael/aldol reaction. | Organic & biomolecular chemistry 20120614 |
| Executing and rationalizing the synthesis of a difluorinated analogue of a ring-expanded calystegine B2. | The Journal of organic chemistry 20120120 |
| Synthesis of N-alkylated noeurostegines and evaluation of their potential as treatment for Gaucher's disease. | Bioorganic & medicinal chemistry letters 20110301 |
| Synthesis and glycosidase inhibitory activity of noeurostegine-a new and potent inhibitor of beta-glucoside hydrolases. | Organic & biomolecular chemistry 20100121 |
| Electrophoretic determination of calystegines A3 and B2 in potato. | Journal of chromatography. A 20080215 |
| D-glucosamine trimethylene dithioacetal derivatives: formation of six- and seven-membered ring amino carbasugars. Synthesis of (-)-calystegine B3. | Organic & biomolecular chemistry 20071021 |
| Brassicaceae contain nortropane alkaloids. | Phytochemistry 20060901 |
| Alkaloids from the poisonous plant Ipomoea carnea: effects on intracellular lysosomal glycosidase activities in human lymphoblast cultures. | Journal of agricultural and food chemistry 20031217 |
| Glycoalkaloid and calystegine contents of eight potato cultivars. | Journal of agricultural and food chemistry 20030507 |
| A short synthetic route to the calystegine alkaloids. | The Journal of organic chemistry 20030321 |
| Synthesis of enantiomerically pure, highly functionalized, medium-sized carbocycles from carbohydrates: formal total synthesis of (+)-calystegine b(2). | The Journal of organic chemistry 20020531 |
| Synthesis and evaluation of calystegine B2 analogues as glycosidase inhibitors. | The Journal of organic chemistry 20011116 |
Related Products
© 2019 Angene International Limited. All rights Reserved.