200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 127393-89-9
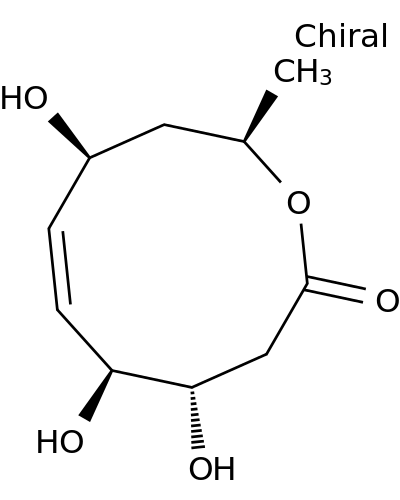
127393-89-9 | 2H-Oxecin-2-one, 3,4,5,8,9,10-hexahydro-4,5,8-trihydroxy-10-methyl-, (4S,5S,6E,8S,10R)-
CAS No: 127393-89-9 Catalog No: AG000X1E MDL No:
Product Description
Catalog Number:
AG000X1E
Chemical Name:
2H-Oxecin-2-one, 3,4,5,8,9,10-hexahydro-4,5,8-trihydroxy-10-methyl-, (4S,5S,6E,8S,10R)-
CAS Number:
127393-89-9
Molecular Formula:
C10H16O5
Molecular Weight:
216.2310
IUPAC Name:
(2R,4S,7S,8S)-4,7,8-trihydroxy-2-methyl-2,3,4,7,8,9-hexahydrooxecin-10-one
InChI:
InChI=1S/C10H16O5/c1-6-4-7(11)2-3-8(12)9(13)5-10(14)15-6/h2-3,6-9,11-13H,4-5H2,1H3/t6-,7-,8+,9+/m1/s1
InChI Key:
HWMMWMJBUOCCFZ-HXFLIBJXSA-N
SMILES:
O[C@@H]1/C=C/[C@H](O)[C@@H](O)CC(=O)O[C@@H](C1)C
Properties
Complexity:
250
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
216.1g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
216.233g/mol
Monoisotopic Mass:
216.1g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
87A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
1
XLogP3:
-0.8
Literature
| Title | Journal |
|---|---|
| Asymmetric synthesis of allylsilanes by the borylation of lithiated carbamates: formal total synthesis of (-)-decarestrictine D. | Angewandte Chemie (International ed. in English) 20100607 |
| Botryolides A-E, decarestrictine analogues from a fungicolous Botryotrichum sp. (NRRL 38180). | Journal of natural products 20080301 |
| Nickel-catalyzed coupling producing (2Z)-2,4-alkadien-1-ols, conversion to (E)-3-alkene-1,2,5-triol derivatives, and synthesis of decarestrictine D. | The Journal of organic chemistry 20070302 |
Related Products
© 2019 Angene International Limited. All rights Reserved.