200,000+ products from a single source!
sales@angenechem.com
Home > Fluorides > 127127-25-7
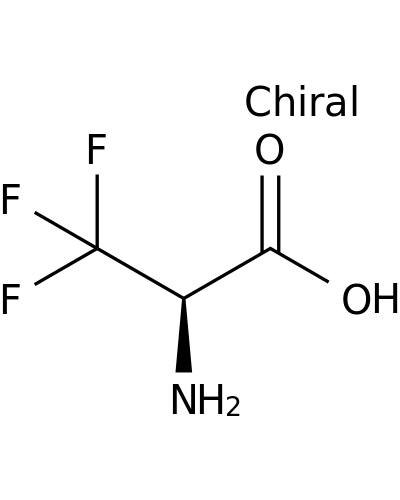
127127-25-7 | L-Alanine, 3,3,3-trifluoro-
CAS No: 127127-25-7 Catalog No: AG000WOI MDL No:
Product Description
Catalog Number:
AG000WOI
Chemical Name:
L-Alanine, 3,3,3-trifluoro-
CAS Number:
127127-25-7
Molecular Formula:
C3H4F3NO2
Molecular Weight:
143.0646
IUPAC Name:
(2R)-2-amino-3,3,3-trifluoropropanoic acid
InChI:
InChI=1S/C3H4F3NO2/c4-3(5,6)1(7)2(8)9/h1H,7H2,(H,8,9)/t1-/m1/s1
InChI Key:
HMJQKIDUCWWIBW-PVQJCKRUSA-N
SMILES:
N[C@@H](C(F)(F)F)C(=O)O
UNII:
NT6AV26MM7
Properties
Complexity:
121
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
143.019g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
143.065g/mol
Monoisotopic Mass:
143.019g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
63.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-2.3
Literature
| Title | Journal |
|---|---|
| Low energy (0-10 eV) electron driven reactions in the halogenated organic acids CCl3COOH, CClF2COOH, and CF3CHNH2COOH (trifluoroalanine). | The Journal of chemical physics 20110928 |
| The role of fluorine in the stereoselective tandem aza-Michael addition to acrylamide acceptors: an experimental and theoretical mechanistic study. | Chemistry (Weinheim an der Bergstrasse, Germany) 20070101 |
| Methyltrifluoropyruvate imines possessing N-oxalyl and N-phosphonoformyl groups--precursors to a variety of alpha-CF3-alpha-amino acid derivatives. | Organic & biomolecular chemistry 20061007 |
| Suicide inhibition of alpha-oxamine synthases: structures of the covalent adducts of 8-amino-7-oxononanoate synthase with trifluoroalanine. | Organic & biomolecular chemistry 20060407 |
| A novel route to dipeptides via noncondensation of amino acids: 2-aminoperfluoropropene as a synthon for trifluoroalanine dipeptides. | Organic letters 20060302 |
| Highly stereoselective tandem aza-Michael addition-enolate protonation to form partially modified retropeptide mimetics incorporating a trifluoroalanine surrogate. | Angewandte Chemie (International ed. in English) 20030509 |
| Design of radical-resistant amino acid residues: a combined theoretical and experimental investigation. | Journal of the American Chemical Society 20030409 |
| Identification and role of ionizing functional groups at the active center of Rhodotorula gracilis D-amino acid oxidase. | FEBS letters 20011102 |
Related Products
© 2019 Angene International Limited. All rights Reserved.