200,000+ products from a single source!
sales@angenechem.com
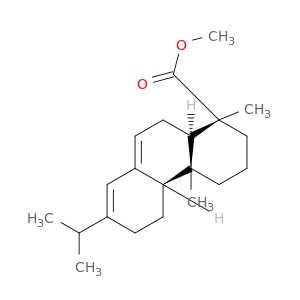
127-25-3 | 1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,4b,5,6,10,10a-decahydro-1,4a-dimethyl-7-(1-methylethyl)-, methyl ester, (1R,4aR,4bR,10aR)-
CAS No: 127-25-3 Catalog No: AG000WJ0 MDL No:
Product Description
Catalog Number:
AG000WJ0
Chemical Name:
1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,4b,5,6,10,10a-decahydro-1,4a-dimethyl-7-(1-methylethyl)-, methyl ester, (1R,4aR,4bR,10aR)-
CAS Number:
127-25-3
Molecular Formula:
C21H32O2
Molecular Weight:
316.4776
IUPAC Name:
methyl (1R,4aR,4bR,10aR)-1,4a-dimethyl-7-propan-2-yl-2,3,4,4b,5,6,10,10a-octahydrophenanthrene-1-carboxylate
InChI:
InChI=1S/C21H32O2/c1-14(2)15-7-9-17-16(13-15)8-10-18-20(17,3)11-6-12-21(18,4)19(22)23-5/h8,13-14,17-18H,6-7,9-12H2,1-5H3/t17-,18+,20+,21+/m0/s1
InChI Key:
OVXRPXGVKBHGQO-UYWIDEMCSA-N
SMILES:
COC(=O)[C@]1(C)CCC[C@]2([C@H]1CC=C1[C@@H]2CCC(=C1)C(C)C)C
EC Number:
204-832-7
UNII:
BY54I7IT3L
NSC Number:
2141
Properties
Complexity:
556
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
0
Exact Mass:
316.24g/mol
Formal Charge:
0
Heavy Atom Count:
23
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
316.485g/mol
Monoisotopic Mass:
316.24g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
26.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
5.1
Literature
| Title | Journal |
|---|---|
| Synthesis and biological evaluation of dehydroabietic acid derivatives. | European journal of medicinal chemistry 20100201 |
| Synthesis and biological evaluation of abietic acid derivatives. | European journal of medicinal chemistry 20090601 |
| Chemical composition and biological activity of a new type of Brazilian propolis: red propolis. | Journal of ethnopharmacology 20070905 |
| Oxidation products of abietic acid and its methyl ester. | Journal of natural products 20021101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.