200,000+ products from a single source!
sales@angenechem.com
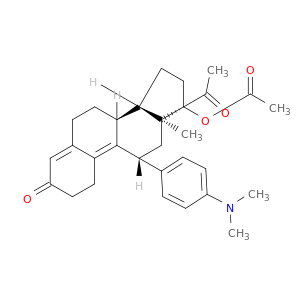
126784-99-4 | (11β)-17-(Acetyloxy)-11-[4-(dimethylamino)phenyl]-19-norpregna-4,9-diene-3,20-dione
CAS No: 126784-99-4 Catalog No: AG003AC7 MDL No:MFCD00899035
Product Description
Catalog Number:
AG003AC7
Chemical Name:
(11β)-17-(Acetyloxy)-11-[4-(dimethylamino)phenyl]-19-norpregna-4,9-diene-3,20-dione
CAS Number:
126784-99-4
Molecular Formula:
C30H37NO4
Molecular Weight:
475.6191
MDL Number:
MFCD00899035
IUPAC Name:
[(8S,11R,13S,14S,17R)-17-acetyl-11-[4-(dimethylamino)phenyl]-13-methyl-3-oxo-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl] acetate
InChI:
InChI=1S/C30H37NO4/c1-18(32)30(35-19(2)33)15-14-27-25-12-8-21-16-23(34)11-13-24(21)28(25)26(17-29(27,30)3)20-6-9-22(10-7-20)31(4)5/h6-7,9-10,16,25-27H,8,11-15,17H2,1-5H3/t25-,26+,27-,29-,30-/m0/s1
InChI Key:
OOLLAFOLCSJHRE-ZHAKMVSLSA-N
SMILES:
CC(=O)O[C@@]1(CC[C@@H]2[C@]1(C)C[C@H](c1ccc(cc1)N(C)C)C1=C3CCC(=O)C=C3CC[C@@H]21)C(=O)C
UNII:
YF7V70N02B
Properties
Complexity:
984
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
5
Defined Bond Stereocenter Count:
0
Exact Mass:
475.272g/mol
Formal Charge:
0
Heavy Atom Count:
35
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
475.629g/mol
Monoisotopic Mass:
475.272g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
63.7A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.5
Literature
| Title | Journal |
|---|---|
| Ulipristal acetate - safety and pharmacokinetics following multiple doses of 10-50 mg per day. | Journal of clinical pharmacy and therapeutics 20130801 |
| Emergency contraception. | Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology 20130301 |
| Results from pooled Phase III studies of ulipristal acetate for emergency contraception. | Contraception 20121201 |
| Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. | International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists 20121101 |
| Emergency contraception: separating fact from fiction. | Cleveland Clinic journal of medicine 20121101 |
| Ulipristal acetate does not impact human normal breast tissue. | Human reproduction (Oxford, England) 20120901 |
| Ulipristal acetate as an emergency contraceptive agent. | Expert opinion on pharmacotherapy 20120901 |
| Ulipristal acetate and its role in emergency contraception: a comment. | Expert opinion on pharmacotherapy 20120901 |
| Interventions for emergency contraception. | The Cochrane database of systematic reviews 20120815 |
| Ulipristal acetate: a novel option for the medical management of symptomatic uterine fibroids. | Advances in therapy 20120801 |
| Pharmacokinetic evaluation of ulipristal acetate for uterine leiomyoma treatment. | Expert opinion on drug metabolism & toxicology 20120701 |
| Emergency contraception: an underutilized resource. | The Journal of family practice 20120701 |
| Ulipristal acetate: in uterine fibroids. | Drugs 20120528 |
| Effects of a novel estrogen-free, progesterone receptor modulator contraceptive vaginal ring on inhibition of ovulation, bleeding patterns and endometrium in normal women. | Contraception 20120501 |
| Ulipristal acetate versus placebo for fibroid treatment before surgery. | The New England journal of medicine 20120202 |
| Ulipristal acetate versus leuprolide acetate for uterine fibroids. | The New England journal of medicine 20120202 |
| Uterine fibroids and evidence-based medicine--not an oxymoron. | The New England journal of medicine 20120202 |
| Ulipristal acetate: review of the efficacy and safety of a newly approved agent for emergency contraception. | Clinical therapeutics 20120101 |
| Ulipristal acetate: the newest emergency contraceptive. | Nursing for women's health 20120101 |
| Current and future medical treatments for menometrorrhagia during the premenopause. | Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology 20111201 |
| [Forgetting hormonal contraceptive methods: expert opinion about their daily management in clinical routine practice]. | Gynecologie, obstetrique & fertilite 20111101 |
| Adolescents and emergency contraception: update 2011. | Current opinion in obstetrics & gynecology 20111001 |
| Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. | Contraception 20111001 |
| Unintended pregnancy in Australia: what more can we do? | The Medical journal of Australia 20110815 |
| Ulipristal acetate: a new emergency contraceptive. | Expert review of clinical pharmacology 20110701 |
| New drugs 2011 part 2. | Nursing 20110601 |
| Ulipristal acetate: contraceptive or contragestive? | The Annals of pharmacotherapy 20110601 |
| Ulipristal acetate for emergency contraception. | The Annals of pharmacotherapy 20110601 |
| Ulipristal acetate: a review of its use in emergency contraception. | Drugs 20110507 |
| LNG may still be the most cost-effective oral emergency contraception method. | The journal of family planning and reproductive health care 20110401 |
| Ella: a new emergency contraceptive. | The Medical letter on drugs and therapeutics 20110110 |
| Ulipristal acetate: a new emergency contraceptive that is safe and more effective than levonorgestrel. | Women's health (London, England) 20110101 |
| New drugs: dabigatran etexilate mesylate, fingolimod hydrochloride, and ulipristal acetate. | Journal of the American Pharmacists Association : JAPhA 20110101 |
| The state-of-the-art of emergency contraception with the cutting edge drug. | German medical science : GMS e-journal 20110101 |
| [Ulipristal acetate: an emergency contraception extended to 5 days using a progesterone receptor modulator (Ellaone)]. | Revue medicale de Liege 20101201 |
| Mechanism of action of emergency contraception. | Contraception 20101101 |
| Ulipristal acetate taken 48-120 hours after intercourse for emergency contraception. | Obstetrics and gynecology 20100201 |
| 11-(pyridinylphenyl)steroids--a new class of mixed-profile progesterone agonists/antagonists. | Bioorganic & medicinal chemistry 20080315 |
| In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. | The Journal of steroid biochemistry and molecular biology 20040301 |
Related Products
© 2019 Angene International Limited. All rights Reserved.