200,000+ products from a single source!
sales@angenechem.com
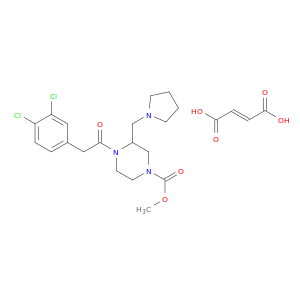
126766-32-3 | 1-Piperazinecarboxylic acid, 4-[2-(3,4-dichlorophenyl)acetyl]-3-(1-pyrrolidinylmethyl)-, methyl ester, (2E)-2-butenedioate (1:1)
CAS No: 126766-32-3 Catalog No: AG000T7X MDL No:
Product Description
Catalog Number:
AG000T7X
Chemical Name:
1-Piperazinecarboxylic acid, 4-[2-(3,4-dichlorophenyl)acetyl]-3-(1-pyrrolidinylmethyl)-, methyl ester, (2E)-2-butenedioate (1:1)
CAS Number:
126766-32-3
Molecular Formula:
C23H29Cl2N3O7
Molecular Weight:
530.3983
IUPAC Name:
(E)-but-2-enedioic acid;methyl 4-[2-(3,4-dichlorophenyl)acetyl]-3-(pyrrolidin-1-ylmethyl)piperazine-1-carboxylate
InChI:
InChI=1S/C19H25Cl2N3O3.C4H4O4/c1-27-19(26)23-8-9-24(15(13-23)12-22-6-2-3-7-22)18(25)11-14-4-5-16(20)17(21)10-14;5-3(6)1-2-4(7)8/h4-5,10,15H,2-3,6-9,11-13H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1+
InChI Key:
ABTNETSDXZBJTE-WLHGVMLRSA-N
SMILES:
OC(=O)/C=C/C(=O)O.COC(=O)N1CCN(C(C1)CN1CCCC1)C(=O)Cc1ccc(c(c1)Cl)Cl
Properties
Complexity:
649
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
2
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
1
Exact Mass:
529.138g/mol
Formal Charge:
0
Heavy Atom Count:
35
Hydrogen Bond Acceptor Count:
8
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
530.399g/mol
Monoisotopic Mass:
529.138g/mol
Rotatable Bond Count:
7
Topological Polar Surface Area:
128A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Discovery of aminobenzyloxyarylamides as κ opioid receptor selective antagonists: application to preclinical development of a κ opioid receptor antagonist receptor occupancy tracer. | Journal of medicinal chemistry 20111208 |
| Pharmacological characterization of 2-methyl-N-((2'-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine (PF-04455242), a high-affinity antagonist selective for κ-opioid receptors. | The Journal of pharmacology and experimental therapeutics 20111101 |
| [11C]GR103545: novel one-pot radiosynthesis with high specific activity. | Nuclear medicine and biology 20110201 |
| Effects of atypical kappa-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates. | The Journal of pharmacology and experimental therapeutics 20090101 |
| Myocardial resistance to ischemic and reperfusion injuries under conditions of chronic administration of opioid receptor agonists and antagonists. | Bulletin of experimental biology and medicine 20080601 |
| Opioid regulation of spinal cord plasticity: evidence the kappa-2 opioid receptor agonist GR89696 inhibits learning within the rat spinal cord. | Neurobiology of learning and memory 20080101 |
| [Role of kappa-opioid receptors in the regulation of cardiac resistance to arrhythmogenic effects of ischemia and reperfusion]. | Rossiiskii fiziologicheskii zhurnal imeni I.M. Sechenova 20061201 |
| Induction of bladder sphincter dyssynergia by kappa-2 opioid receptor agonists in the female rat. | The Journal of urology 20040101 |
| [(11)C]-GR89696, a potent kappa opiate receptor radioligand; in vivo binding of the R and S enantiomers. | Nuclear medicine and biology 20020101 |
| GR89,696: a potent kappa-opioid agonist with subtype selectivity in rhesus monkeys. | The Journal of pharmacology and experimental therapeutics 20010901 |
| Methylated analogues of methyl (R)-4-(3,4-dichlorophenylacetyl)- 3-(pyrrolidin-1-ylmethyl)piperazine-1-carboxylate (GR-89,696) as highly potent kappa-receptor agonists: stereoselective synthesis, opioid-receptor affinity, receptor selectivity, and functional studies. | Journal of medicinal chemistry 20010816 |
| Synthesis and stereoselective kappa-receptor binding of methylated analogues of GR-89.696. | European journal of medicinal chemistry 20010201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







