200,000+ products from a single source!
sales@angenechem.com
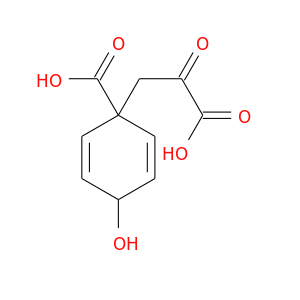
126-49-8 | 2,5-Cyclohexadiene-1-propanoic acid, 1-carboxy-4-hydroxy-α-oxo-
CAS No: 126-49-8 Catalog No: AG000QNU MDL No:
Product Description
Catalog Number:
AG000QNU
Chemical Name:
2,5-Cyclohexadiene-1-propanoic acid, 1-carboxy-4-hydroxy-α-oxo-
CAS Number:
126-49-8
Molecular Formula:
C10H10O6
Molecular Weight:
226.1828
IUPAC Name:
1-(2-carboxy-2-oxoethyl)-4-hydroxycyclohexa-2,5-diene-1-carboxylic acid
InChI:
InChI=1S/C10H10O6/c11-6-1-3-10(4-2-6,9(15)16)5-7(12)8(13)14/h1-4,6,11H,5H2,(H,13,14)(H,15,16)
InChI Key:
FPWMCUPFBRFMLH-UHFFFAOYSA-N
SMILES:
OC1C=CC(C=C1)(CC(=O)C(=O)O)C(=O)O
UNII:
Z66B98Z97I
Properties
Complexity:
378
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
226.048g/mol
Formal Charge:
0
Heavy Atom Count:
16
Hydrogen Bond Acceptor Count:
6
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
226.184g/mol
Monoisotopic Mass:
226.048g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
112A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.5
Literature
| Title | Journal |
|---|---|
| Structure-guided discovery of the metabolite carboxy-SAM that modulates tRNA function. | Nature 20130606 |
| Characterisation of volatile and non-volatile metabolites in etiolated leaves of tea (Camellia sinensis) plants in the dark. | Food chemistry 20121215 |
| Molecular dynamics simulation of the last step of a catalytic cycle: product release from the active site of the enzyme chorismate mutase from Mycobacterium tuberculosis. | Protein science : a publication of the Protein Society 20121101 |
| Stereochemical outcome at four stereogenic centers during conversion of prephenate to tetrahydrotyrosine by BacABGF in the bacilysin pathway. | Biochemistry 20120717 |
| Olefin isomerization regiochemistries during tandem action of BacA and BacB on prephenate in bacilysin biosynthesis. | Biochemistry 20120417 |
| Crystal structure of prephenate dehydrogenase from Streptococcus mutans. | International journal of biological macromolecules 20111101 |
| An N-terminal protein degradation tag enables robust selection of highly active enzymes. | Biochemistry 20111011 |
| Dihydrophenylalanine: a prephenate-derived Photorhabdus luminescens antibiotic and intermediate in dihydrostilbene biosynthesis. | Chemistry & biology 20110923 |
| Complementation of the pha2 yeast mutant suggests functional differences for arogenate dehydratases from Arabidopsis thaliana. | Plant physiology and biochemistry : PPB 20110801 |
| Identification of genes in the phenylalanine metabolic pathway by ectopic expression of a MYB transcription factor in tomato fruit. | The Plant cell 20110701 |
| L-Tryptophan Production by Auxotrophic and Analogue Resistant Mutants of Aureobacterium flavescens. | International journal of tryptophan research : IJTR 20110101 |
| Prephenate decarboxylases: a new prephenate-utilizing enzyme family that performs nonaromatizing decarboxylation en route to diverse secondary metabolites. | Biochemistry 20101026 |
| Investigation of anticapsin biosynthesis reveals a four-enzyme pathway to tetrahydrotyrosine in Bacillus subtilis. | Biochemistry 20100209 |
| Role of Bacillus subtilis BacB in the synthesis of bacilysin. | The Journal of biological chemistry 20091113 |
| Mechanism and plasticity of isochorismate pyruvate lyase: a computational study. | Journal of the American Chemical Society 20091111 |
| Genetic and biochemical identification of the chorismate mutase from Corynebacterium glutamicum. | Microbiology (Reading, England) 20091001 |
| On-the-path random walk sampling for efficient optimization of minimum free-energy path. | Journal of computational chemistry 20090801 |
| Phenylalanine biosynthesis in Arabidopsis thaliana. Identification and characterization of arogenate dehydratases. | The Journal of biological chemistry 20071019 |
| Vitamin K1 accumulation in tobacco plants overexpressing bacterial genes involved in the biosynthesis of salicylic acid. | Journal of biotechnology 20070130 |
| Computer-aided rational design of catalytic antibodies: The 1F7 case. | Angewandte Chemie (International ed. in English) 20070101 |
| Multiple-steering QM-MM calculation of the free energy profile in chorismate mutase. | Journal of the American Chemical Society 20050518 |
| Investigation of ligand binding and protein dynamics in Bacillus subtilis chorismate mutase by transverse relaxation optimized spectroscopy-nuclear magnetic resonance. | Biochemistry 20050510 |
| Differential transition-state stabilization in enzyme catalysis: quantum chemical analysis of interactions in the chorismate mutase reaction and prediction of the optimal catalytic field. | Journal of the American Chemical Society 20041215 |
| Just a near attack conformer for catalysis (chorismate to prephenate rearrangements in water, antibody, enzymes, and their mutants). | Journal of the American Chemical Society 20030903 |
| Investigation of solvent effects for the Claisen rearrangement of chorismate to prephenate: mechanistic interpretation via near attack conformations. | Journal of the American Chemical Society 20030604 |
| Comparison of formation of reactive conformers (NACs) for the Claisen rearrangement of chorismate to prephenate in water and in the E. coli mutase: the efficiency of the enzyme catalysis. | Journal of the American Chemical Society 20030514 |
| Efficiency of lignin biosynthesis: a quantitative analysis. | Annals of botany 20030501 |
| Understanding the role of active-site residues in chorismate mutase catalysis from molecular-dynamics simulations. | Angewandte Chemie (International ed. in English) 20030404 |
| Mapping of chorismate mutase and prephenate dehydrogenase domains in the Escherichia coli T-protein. | European journal of biochemistry 20030201 |
| The emerging periplasm-localized subclass of AroQ chorismate mutases, exemplified by those from Salmonella typhimurium and Pseudomonas aeruginosa. | Genome biology 20010101 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







