200,000+ products from a single source!
sales@angenechem.com
Home > Nitro Compounds > 126-11-4
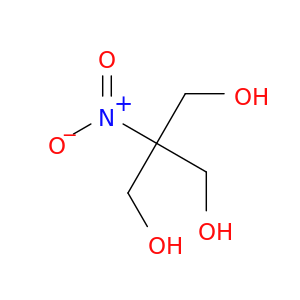
126-11-4 | 1,3-Propanediol, 2-(hydroxymethyl)-2-nitro-
CAS No: 126-11-4 Catalog No: AG000QOA MDL No:MFCD00007395
Product Description
Catalog Number:
AG000QOA
Chemical Name:
1,3-Propanediol, 2-(hydroxymethyl)-2-nitro-
CAS Number:
126-11-4
Molecular Formula:
C4H9NO5
Molecular Weight:
151.1180
MDL Number:
MFCD00007395
IUPAC Name:
2-(hydroxymethyl)-2-nitropropane-1,3-diol
InChI:
InChI=1S/C4H9NO5/c6-1-4(2-7,3-8)5(9)10/h6-8H,1-3H2
InChI Key:
OLQJQHSAWMFDJE-UHFFFAOYSA-N
SMILES:
OCC([N+](=O)[O-])(CO)CO
EC Number:
204-769-5
UNII:
3E794G4ZRB
Properties
Complexity:
107
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
151.048g/mol
Formal Charge:
0
Heavy Atom Count:
10
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
151.118g/mol
Monoisotopic Mass:
151.048g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
107A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-2.2
Literature
| Title | Journal |
|---|---|
| Synthesis of tris-hydroxymethyl-based nitrone derivatives with highly reactive nitronyl carbon. | The Journal of organic chemistry 20120120 |
| Bioactive compounds and biological activities of Jatropha curcas L. kernel meal extract. | International journal of molecular sciences 20110101 |
| Formaldehyde-releasers: relationship to formaldehyde contact allergy. Part 2: Metalworking fluids and remainder. | Contact dermatitis 20100901 |
| Efficient synthesis of a peculiar vicinal diamine semiochemical from Streptomyces natalensis. | Organic letters 20100806 |
| Aliphatic beta-nitroalcohols for therapeutic corneoscleral cross-linking: chemical mechanisms and higher order nitroalcohols. | Investigative ophthalmology & visual science 20100201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.