200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 125412-70-6
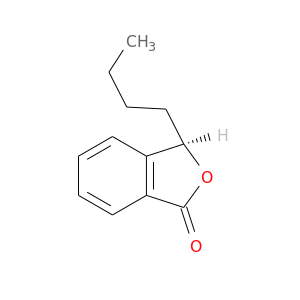
125412-70-6 | 1(3H)-Isobenzofuranone, 3-butyl-, (3R)-
CAS No: 125412-70-6 Catalog No: AG000NNV MDL No:
Product Description
Catalog Number:
AG000NNV
Chemical Name:
1(3H)-Isobenzofuranone, 3-butyl-, (3R)-
CAS Number:
125412-70-6
Molecular Formula:
C12H14O2
Molecular Weight:
190.2384
IUPAC Name:
(3R)-3-butyl-3H-2-benzofuran-1-one
InChI:
InChI=1S/C12H14O2/c1-2-3-8-11-9-6-4-5-7-10(9)12(13)14-11/h4-7,11H,2-3,8H2,1H3/t11-/m1/s1
InChI Key:
HJXMNVQARNZTEE-LLVKDONJSA-N
SMILES:
CCCC[C@H]1OC(=O)c2c1cccc2
UNII:
9465GZ4W47
Properties
Complexity:
212
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
190.099g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
190.242g/mol
Monoisotopic Mass:
190.099g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
26.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.8
Literature
| Title | Journal |
|---|---|
| An efficient system for the asymmetric acylation of (R,S)-3-n-butylphthalide catalyzed by novozyme 435. | Preparative biochemistry & biotechnology 20100101 |
| Novozyme 435-catalyzed asymmetric acylation of (R, S)-3-n- butylphthalide in hexane. | Preparative biochemistry & biotechnology 20090101 |
| Synthesis, resolution, and antiplatelet activity of 3-substituted 1(3H)-isobenzofuranone. | Bioorganic & medicinal chemistry letters 20070915 |
Related Products
© 2019 Angene International Limited. All rights Reserved.