200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1254-03-1
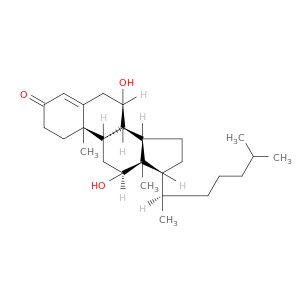
1254-03-1 | Cholest-4-en-3-one, 7,12-dihydroxy-, (7α,12α)-
CAS No: 1254-03-1 Catalog No: AG000NMM MDL No:
Product Description
Catalog Number:
AG000NMM
Chemical Name:
Cholest-4-en-3-one, 7,12-dihydroxy-, (7α,12α)-
CAS Number:
1254-03-1
Molecular Formula:
C27H44O3
Molecular Weight:
416.6365
IUPAC Name:
(7R,8R,9S,10R,12S,13R,14S,17R)-7,12-dihydroxy-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one
InChI:
InChI=1S/C27H44O3/c1-16(2)7-6-8-17(3)20-9-10-21-25-22(15-24(30)27(20,21)5)26(4)12-11-19(28)13-18(26)14-23(25)29/h13,16-17,20-25,29-30H,6-12,14-15H2,1-5H3/t17-,20-,21+,22+,23-,24+,25+,26+,27-/m1/s1
InChI Key:
UQPYXHJTHPHOMM-NIBOIBLTSA-N
SMILES:
CC(CCC[C@H]([C@H]1CC[C@@H]2[C@]1(C)[C@@H](O)C[C@H]1[C@H]2[C@H](O)CC2=CC(=O)CC[C@]12C)C)C
Properties
Complexity:
696
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
9
Defined Bond Stereocenter Count:
0
Exact Mass:
416.329g/mol
Formal Charge:
0
Heavy Atom Count:
30
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
416.646g/mol
Monoisotopic Mass:
416.329g/mol
Rotatable Bond Count:
5
Topological Polar Surface Area:
57.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
6
Literature
| Title | Journal |
|---|---|
| Crystal structures of human Delta4-3-ketosteroid 5beta-reductase (AKR1D1) reveal the presence of an alternative binding site responsible for substrate inhibition. | Biochemistry 20081223 |
| The crystal structure of human Delta4-3-ketosteroid 5beta-reductase defines the functional role of the residues of the catalytic tetrad in the steroid double bond reduction mechanism. | Biochemistry 20080812 |
| Differences in hepatic levels of intermediates in bile acid biosynthesis between Cyp27(-/-) mice and CTX. | Journal of lipid research 20010201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.