200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 124-42-5
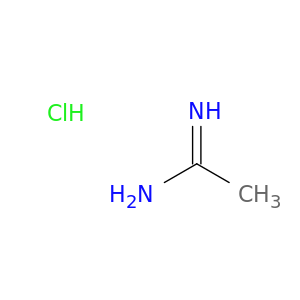
124-42-5 | Ethanimidamide, hydrochloride (1:1)
CAS No: 124-42-5 Catalog No: AG000LD7 MDL No:MFCD00013016
Product Description
Catalog Number:
AG000LD7
Chemical Name:
Ethanimidamide, hydrochloride (1:1)
CAS Number:
124-42-5
Molecular Formula:
C2H7ClN2
Molecular Weight:
94.5434
MDL Number:
MFCD00013016
IUPAC Name:
ethanimidamide;hydrochloride
InChI:
InChI=1S/C2H6N2.ClH/c1-2(3)4;/h1H3,(H3,3,4);1H
InChI Key:
WCQOBLXWLRDEQA-UHFFFAOYSA-N
SMILES:
CC(=N)N.Cl
EC Number:
204-700-9
UNII:
M2026K4JCF
NSC Number:
7595
Properties
Complexity:
31
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
2
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
94.03g/mol
Formal Charge:
0
Heavy Atom Count:
5
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
94.542g/mol
Monoisotopic Mass:
94.03g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
49.9A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
Literature
| Title | Journal |
|---|---|
| Novel aminobenzyl-acetamidine derivative modulate the differential regulation of NOSs in LPS induced inflammatory response: role of PI3K/Akt pathway. | Biochimica et biophysica acta 20121201 |
| Selective inhibition of inducible nitric oxide synthase by derivatives of acetamidine. | Medicinal chemistry (Shariqah (United Arab Emirates)) 20121101 |
| A mild liquid reduction route toward uniform blue-emitting EuCl2 nanoprisms and nanorods. | Inorganic chemistry 20110801 |
| Selective inhibition of iNOS by benzyl- and dibenzyl derivatives of N-(3-aminobenzyl)acetamidine. | ChemMedChem 20110704 |
| A synthesis of acetamidines. | The Journal of organic chemistry 20110318 |
| Characterization and inactivation of an agmatine deiminase from Helicobacter pylori. | Bioorganic chemistry 20100401 |
| Haloacetamidine-based inactivators of protein arginine deiminase 4 (PAD4): evidence that general acid catalysis promotes efficient inactivation. | Chembiochem : a European journal of chemical biology 20100125 |
| Four-component synthesis of fully substituted 1,2,4-triazoles. | Angewandte Chemie (International ed. in English) 20100101 |
| Highly efficient copper-catalyzed cascade synthesis of quinazoline and quinazolinone derivatives. | Chemical communications (Cambridge, England) 20081221 |
| Differential effects of selective and non-selective inhibition of nitric oxide synthase on the expression and activity of cyclooxygenase-2 during gastric ulcer healing. | European journal of pharmacology 20060501 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







