200,000+ products from a single source!
sales@angenechem.com
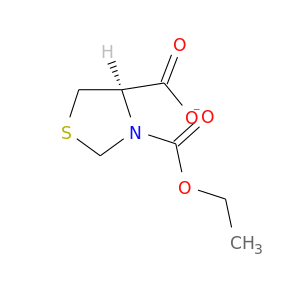
122946-43-4 | 3,4-Thiazolidinedicarboxylic acid, 3-ethyl ester, (4R)-
CAS No: 122946-43-4 Catalog No: AG000JT9 MDL No:MFCD00867667
Product Description
Catalog Number:
AG000JT9
Chemical Name:
3,4-Thiazolidinedicarboxylic acid, 3-ethyl ester, (4R)-
CAS Number:
122946-43-4
Molecular Formula:
C7H10NO4S-
Molecular Weight:
204.2236
MDL Number:
MFCD00867667
IUPAC Name:
(4R)-3-ethoxycarbonyl-1,3-thiazolidine-4-carboxylic acid
InChI:
InChI=1S/C7H11NO4S/c1-2-12-7(11)8-4-13-3-5(8)6(9)10/h5H,2-4H2,1H3,(H,9,10)/t5-/m0/s1
InChI Key:
XBJWOGLKABXFJE-YFKPBYRVSA-N
SMILES:
[O-]C(=O)[C@@H]1CSCN1C(=O)OCC
UNII:
124I3FE35T
Properties
Complexity:
221
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
205.041g/mol
Formal Charge:
0
Heavy Atom Count:
13
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
205.228g/mol
Monoisotopic Mass:
205.041g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
92.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.6
Literature
| Title | Journal |
|---|---|
| Treatment of mild to moderate seborrhoeic dermatitis with MAS064D (Sebclair), a novel topical medical device: results of a pilot, randomized, double-blind, controlled trial. | Journal of the European Academy of Dermatology and Venereology : JEADV 20080301 |
| A double-blind, randomised, vehicle-controlled clinical study to evaluate the efficacy of MAS065D in limiting the effects of radiation on the skin: interim analysis. | European journal of dermatology : EJD 20080101 |
| Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. | Investigative ophthalmology & visual science 20070901 |
| A multicenter, randomized, vehicle-controlled clinical study to examine the efficacy and safety of MAS063DP (Atopiclair) in the management of mild to moderate atopic dermatitis in adults. | Journal of drugs in dermatology : JDD 20060301 |
| A randomised, double-blind, vehicle-controlled study to evaluate the efficacy and safety of MAS063D (Atopiclair) in the treatment of mild to moderate atopic dermatitis. | European journal of dermatology : EJD 20050101 |
| Absorption, distribution, metabolism and excretion of telmesteine, a mucolitic agent, in rat. | Xenobiotica; the fate of foreign compounds in biological systems 20030701 |
Related Products
© 2019 Angene International Limited. All rights Reserved.