200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 122555-04-8
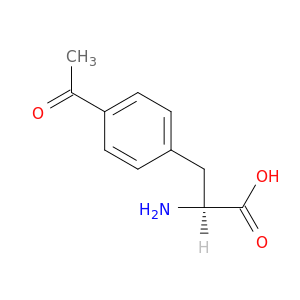
122555-04-8 | L-Phenylalanine, 4-acetyl-
CAS No: 122555-04-8 Catalog No: AG0017G5 MDL No:MFCD06659983
Product Description
Catalog Number:
AG0017G5
Chemical Name:
L-Phenylalanine, 4-acetyl-
CAS Number:
122555-04-8
Molecular Formula:
C11H13NO3
Molecular Weight:
207.2258
MDL Number:
MFCD06659983
IUPAC Name:
(2S)-3-(4-acetylphenyl)-2-aminopropanoic acid
InChI:
InChI=1S/C11H13NO3/c1-7(13)9-4-2-8(3-5-9)6-10(12)11(14)15/h2-5,10H,6,12H2,1H3,(H,14,15)/t10-/m0/s1
InChI Key:
ZXSBHXZKWRIEIA-JTQLQIEISA-N
SMILES:
N[C@H](C(=O)O)Cc1ccc(cc1)C(=O)C
UNII:
Y61IJN1HNY
Properties
Complexity:
244
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
207.09g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
207.229g/mol
Monoisotopic Mass:
207.09g/mol
Rotatable Bond Count:
4
Topological Polar Surface Area:
80.4A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-1.4
Literature
| Title | Journal |
|---|---|
| Synthetic biology: an emerging research field in China. | Biotechnology advances 20111101 |
| Artificial extracellular matrix proteins containing phenylalanine analogues biosynthesized in bacteria using T7 expression system and the PEGylation. | Biomacromolecules 20111010 |
| Designing and engineering of a site-specific incorporation of a keto group in uricase. | Chemical biology & drug design 20110901 |
| Site-specific coupling and sterically controlled formation of multimeric antibody fab fragments with unnatural amino acids. | Journal of molecular biology 20110304 |
| Site-specific labeling of proteins for single-molecule FRET measurements using genetically encoded ketone functionalities. | Methods in molecular biology (Clifton, N.J.) 20110101 |
| Rational design of aminoacyl-tRNA synthetase specific for p-acetyl-L-phenylalanine. | Biochemical and biophysical research communications 20100101 |
| FTIR analysis of GPCR activation using azido probes. | Nature chemical biology 20090601 |
| High-level cell-free synthesis yields of proteins containing site-specific non-natural amino acids. | Biotechnology and bioengineering 20090201 |
| Site-specific incorporation of keto amino acids into functional G protein-coupled receptors using unnatural amino acid mutagenesis. | The Journal of biological chemistry 20080118 |
| An improved system for the generation and analysis of mutant proteins containing unnatural amino acids in Saccharomyces cerevisiae. | Journal of molecular biology 20070803 |
| Leucyl/phenylalanyl(L/F)-tRNA-protein transferase-mediated aminoacyl transfer of a nonnatural amino acid to the N-terminus of peptides and proteins and subsequent functionalization by bioorthogonal reactions. | Biopolymers 20070101 |
| An mRNA-protein fusion at N-terminus for evolutionary protein engineering. | International journal of biological sciences 20070101 |
| Structural characterization of a p-acetylphenylalanyl aminoacyl-tRNA synthetase. | Journal of the American Chemical Society 20051102 |
| American Chemical Society meeting. Unnatural amino acid could prove boon for protein therapeutics. | Science (New York, N.Y.) 20050401 |
| An expanded eukaryotic genetic code. | Science (New York, N.Y.) 20030815 |
| A new strategy for the site-specific modification of proteins in vivo. | Biochemistry 20030610 |
| A designed phenylalanyl-tRNA synthetase variant allows efficient in vivo incorporation of aryl ketone functionality into proteins. | Journal of the American Chemical Society 20020522 |
Related Products
© 2019 Angene International Limited. All rights Reserved.