200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 122540-27-6
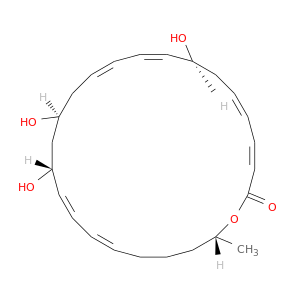
122540-27-6 | Oxacyclotetracosa-3,5,9,11,17,19-hexaen-2-one, 8,14,16-trihydroxy-24-methyl-, (3Z,5E,8S,9E,11Z,14S,16R,17E,19E,24R)-
CAS No: 122540-27-6 Catalog No: AG0017FK MDL No:
Product Description
Catalog Number:
AG0017FK
Chemical Name:
Oxacyclotetracosa-3,5,9,11,17,19-hexaen-2-one, 8,14,16-trihydroxy-24-methyl-, (3Z,5E,8S,9E,11Z,14S,16R,17E,19E,24R)-
CAS Number:
122540-27-6
Molecular Formula:
C24H34O5
Molecular Weight:
402.5238
IUPAC Name:
(3Z,5E,8R,9E,11Z,14S,16S,17E,19E,24R)-8,14,16-trihydroxy-24-methyl-1-oxacyclotetracosa-3,5,9,11,17,19-hexaen-2-one
InChI:
InChI=1S/C24H34O5/c1-20-13-7-3-2-4-8-16-22(26)19-23(27)17-11-5-9-14-21(25)15-10-6-12-18-24(28)29-20/h2,4-6,8-12,14,16,18,20-23,25-27H,3,7,13,15,17,19H2,1H3/b4-2+,10-6+,11-5-,14-9+,16-8+,18-12-/t20-,21+,22-,23+/m1/s1
InChI Key:
XXDIJWSZFWZBRM-QCEWEWFLSA-N
SMILES:
O[C@H]1C/C=C/C=C\C(=O)O[C@H](C)CCC/C=C/C=C/[C@@H](C[C@H](C/C=C\C=C\1)O)O
Properties
Complexity:
627
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
4
Defined Bond Stereocenter Count:
6
Exact Mass:
402.241g/mol
Formal Charge:
0
Heavy Atom Count:
29
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
402.531g/mol
Monoisotopic Mass:
402.241g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
87A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.5
Literature
| Title | Journal |
|---|---|
| Production of bacillomycin- and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. | Journal of agricultural and food chemistry 20120328 |
| Antibacterial activities of macrolactin A and 7-O-succinyl macrolactin A from Bacillus polyfermenticus KJS-2 against vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. | Archives of pharmacal research 20110101 |
| Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. | Microbial cell factories 20090101 |
| Macrolactin is the polyketide biosynthesis product of the pks2 cluster of Bacillus amyloliquefaciens FZB42. | Journal of natural products 20070901 |
| Biological control agent of common scab disease by antagonistic strain Bacillus sp. sunhua. | Journal of applied microbiology 20050101 |
| Archazolid and apicularen: novel specific V-ATPase inhibitors. | BMC biochemistry 20050101 |
| Convergent highly stereoselective preparation of the C12-C24 fragment of macrolactin A. | The Journal of organic chemistry 20040723 |
| Characteristics of the squalene synthase inhibitors produced by a Streptomyces species isolated from soils. | Canadian journal of microbiology 20031101 |
| Design and synthesis of an enzyme-cleavable sensor molecule for phosphodiesterase activity based on fluorescence resonance energy transfer. | Journal of the American Chemical Society 20020227 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







