200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 121733-19-5
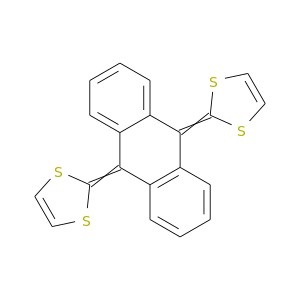
121733-19-5 | 1,3-Dithiole, 2,2'-(9,10-anthracenediylidene)bis-
CAS No: 121733-19-5 Catalog No: AG0015NM MDL No:
Product Description
Catalog Number:
AG0015NM
Chemical Name:
1,3-Dithiole, 2,2'-(9,10-anthracenediylidene)bis-
CAS Number:
121733-19-5
Molecular Formula:
C20H12S4
Molecular Weight:
380.5693
IUPAC Name:
2-[10-(1,3-dithiol-2-ylidene)anthracen-9-ylidene]-1,3-dithiole
InChI:
InChI=1S/C20H12S4/c1-2-6-14-13(5-1)17(19-21-9-10-22-19)15-7-3-4-8-16(15)18(14)20-23-11-12-24-20/h1-12H
InChI Key:
ONZOLRIFSJIOIQ-UHFFFAOYSA-N
SMILES:
C1=CSC(=c2c3ccccc3c(=C3SC=CS3)c3c2cccc3)S1
Properties
Complexity:
538
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
379.982g/mol
Formal Charge:
0
Heavy Atom Count:
24
Hydrogen Bond Acceptor Count:
4
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
380.556g/mol
Monoisotopic Mass:
379.982g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
101A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
5.4
Literature
| Title | Journal |
|---|---|
| Hole-transfer dyads and triads based on perylene monoimide, quaterthiophene, and extended tetrathiafulvalene. | Chemistry (Weinheim an der Bergstrasse, Germany) 20100809 |
| Computational characterization and modeling of buckyball tweezers: density functional study of concave-convex pi...pi interactions. | Physical chemistry chemical physics : PCCP 20080521 |
| Thiolated pi-extended tetrathiafulvalenes: versatile multifunctional pi-systems. | The Journal of organic chemistry 20070216 |
| Studies of potential inversion in an extended tetrathiafulvalene. | Langmuir : the ACS journal of surfaces and colloids 20061205 |
| Extreme conformational constraints in pi-extended tetrathiafulvalenes: unusual topologies and redox behavior of doubly and triply bridged cyclophanes. | Journal of the American Chemical Society 20060816 |
| New strategies and building blocks for functionalised 9,10-bis(1,3-dithiol-2-ylidene)-9,10-dihydroanthracene derivatives, including pyrrolo-annelated derivatives and pi-extended systems with intramolecular charge-transfer. | Organic & biomolecular chemistry 20030207 |
| A (pi-extended tetrathiafulvalene)-fluorene conjugate. Unusual electrochemistry and charge transfer properties: the first observation of a covalent D(2+)-sigma-A(.-) redox state(1). | Journal of the American Chemical Society 20021127 |
| Molecular saddles. 7. New 9,10-bis(1,3-dithiol-2-ylidene)-9,10-dihydroanthracene cyclophanes: synthesis, redox properties, and x-ray crystal structures of neutral species and a dication salt. | The Journal of organic chemistry 20010518 |
| Photochemistry of the pi-extended 9,10-bis(1,3-dithiol-2-ylidene)-9,10-dihydroanthracene system: generation and characterisation of the radical cation, dication, and derived products. | Chemistry (Weinheim an der Bergstrasse, Germany) 20010302 |
| Molecular saddles. 4. Redox-active cyclophanes by bridging the 9,10-bis(1,3-dithiol-2-ylidene)-9,10-dihydroanthracene system: synthesis, electrochemistry, and X-ray crystal structures of neutral species and a dication salt. | The Journal of organic chemistry 20010209 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







