200,000+ products from a single source!
sales@angenechem.com
Home > Nitro Compounds > 121-81-3
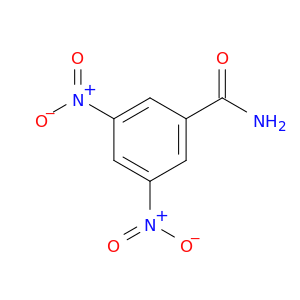
121-81-3 | 3,5-Dinitrobenzamide
CAS No: 121-81-3 Catalog No: AG003IPF MDL No:MFCD00007985
Product Description
Catalog Number:
AG003IPF
Chemical Name:
3,5-Dinitrobenzamide
CAS Number:
121-81-3
Molecular Formula:
C7H5N3O5
Molecular Weight:
211.1317
MDL Number:
MFCD00007985
IUPAC Name:
3,5-dinitrobenzamide
InChI:
InChI=1S/C7H5N3O5/c8-7(11)4-1-5(9(12)13)3-6(2-4)10(14)15/h1-3H,(H2,8,11)
InChI Key:
UUKWKUSGGZNXGA-UHFFFAOYSA-N
SMILES:
NC(=O)c1cc(cc(c1)[N+](=O)[O-])[N+](=O)[O-]
EC Number:
204-499-8
UNII:
9DUJ3CMK8S
Properties
Complexity:
272
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
211.023g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
211.133g/mol
Monoisotopic Mass:
211.023g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
135A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.8
Literature
| Title | Journal |
|---|---|
| Highly sensitive voltammetric sensor based on catechol-derivative-multiwall carbon nanotubes for the catalytic determination of captopril in patient human urine samples. | Colloids and surfaces. B, Biointerfaces 20111015 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Charge-transfer interaction between poly(9-vinylcarbazole) and 3,5-dinitrobenzamido group or 3-nitrobenzamido group. | Langmuir : the ACS journal of surfaces and colloids 20100302 |
| Preparative enantioseparation of (+/-)-N-(3,4-cis-3-decyl-1,2,3,4-tetrahydrophenanthren-4-yl)-3,5-dinitrobenzamide by centrifugal partition chromatography. | Journal of chromatography. A 20100219 |
| Continuous separation of racemic 3,5-dinitrobenzoyl-amino acids in a centrifugal contact separator with the aid of cinchona-based chiral host compounds. | Chemistry (Weinheim an der Bergstrasse, Germany) 20090101 |
| Discrimination of enantiomers of alpha-amino acids by chiral derivatizing reagents from trans-1,2-diaminocyclohexane. | Chirality 20080301 |
| Pi-Pi complexation of bupivacaine and analogues with aromatic receptors: implications for overdose remediation. | International journal of nanomedicine 20070901 |
| A comprehensive chemoselective and enantioselective 2D-HPLC set-up for fast enantiomer analysis of a multicomponent mixture of derivatized amino acids. | Analytical and bioanalytical chemistry 20070801 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Determination of enantiomeric composition by negative-ion electrospray ionization-mass spectrometry using deprotonated N-(3,5-dinitrobenzoyl)amino acids as chiral selectors. | Chirality 20051001 |
| PnrA, a new nitroreductase-family enzyme in the TNT-degrading strain Pseudomonas putida JLR11. | Environmental microbiology 20050801 |
| Elucidation of the chiral recognition mechanism of cinchona alkaloid carbamate-type receptors for 3,5-dinitrobenzoyl amino acids. | Journal of the American Chemical Society 20020724 |
| Solid-phase synthesis of chiral stationary phases based on 2,4,5,6-tetrachloro-1,3-dicyanobenzene derivatives spaced from N-3,5-dinitrobenzoyl alpha-amino acids: comparative study of their resolution efficacy. | Chirality 20010601 |
| Efficacy of 101 antimicrobials and other agents on the development of Cryptosporidium parvum in vitro. | Annals of tropical medicine and parasitology 19961201 |
Related Products
© 2019 Angene International Limited. All rights Reserved.