200,000+ products from a single source!
sales@angenechem.com
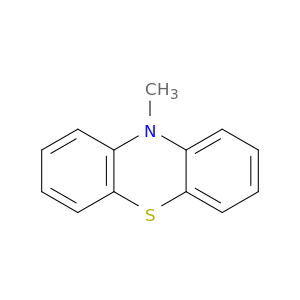
1207-72-3 | 10-Methyl-10H-phenothiazine
CAS No: 1207-72-3 Catalog No: AG003DU8 MDL No:MFCD00041836
Product Description
Catalog Number:
AG003DU8
Chemical Name:
10-Methyl-10H-phenothiazine
CAS Number:
1207-72-3
Molecular Formula:
C13H11NS
Molecular Weight:
213.2981
MDL Number:
MFCD00041836
IUPAC Name:
10-methylphenothiazine
InChI:
InChI=1S/C13H11NS/c1-14-10-6-2-4-8-12(10)15-13-9-5-3-7-11(13)14/h2-9H,1H3
InChI Key:
QXBUYALKJGBACG-UHFFFAOYSA-N
SMILES:
CN1c2ccccc2Sc2c1cccc2
EC Number:
214-896-8
NSC Number:
120
Properties
Complexity:
209
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
213.061g/mol
Formal Charge:
0
Heavy Atom Count:
15
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
213.298g/mol
Monoisotopic Mass:
213.061g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
28.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.2
Literature
| Title | Journal |
|---|---|
| Self sensitized photooxidation of N-methyl phenothiazine: acidity control of the competition between electron and energy transfer mechanisms. | Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology 20121101 |
| 10H-Phenothia-zine 5-oxide. | Acta crystallographica. Section E, Structure reports online 20101201 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| [Electrocatalytic oxidation of glutathione at 10-methylphenothiazine modified carbon paste electrode and its practical analytical application]. | Yao xue xue bao = Acta pharmaceutica Sinica 20080301 |
| Phenothiazine as a redox-active DNA base substitute: comparison with phenothiazine-modified uridine. | Organic & biomolecular chemistry 20080107 |
| The singlet oxygen oxidation of chlorpromazine and some phenothiazine derivatives. Products and reaction mechanisms. | The Journal of organic chemistry 20070720 |
| High energy and quantum efficiency in photoinduced charge separation. | Journal of the American Chemical Society 20070117 |
| Experimental and quantum chemical study on the vibrational spectroscopy of N-methylphenothiazines: part 1. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20060201 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Mechanism of the oxidation of aromatic sulfides catalysed by a water soluble iron porphyrin. | Organic & biomolecular chemistry 20030121 |
| Synthesis and characterization of pi-stacked phenothiazine-labeled oligodeoxynucleotides. | Organic letters 20021226 |
| Synthesis, cyclic voltammetric studies, and electrogenerated chemiluminescence of a new donor-acceptor molecule: 3,7-[Bis[4-phenyl-2-quinolyl]]-10-methylphenothiazine. | Journal of the American Chemical Society 20010919 |
| Synthesis and biological activity of N-acylphenothiazines. | International journal of antimicrobial agents 20000401 |
Related Products
© 2019 Angene International Limited. All rights Reserved.







