200,000+ products from a single source!
sales@angenechem.com
Home > Aldehydes > 120450-87-5
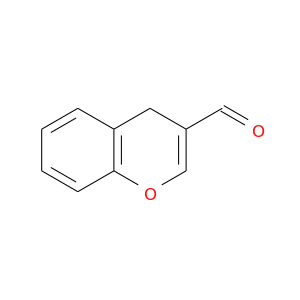
120450-87-5 | 4H-1-Benzopyran-3-carboxaldehyde
CAS No: 120450-87-5 Catalog No: AG00810U MDL No:
Product Description
Catalog Number:
AG00810U
Chemical Name:
4H-1-Benzopyran-3-carboxaldehyde
CAS Number:
120450-87-5
Molecular Formula:
C10H8O2
Molecular Weight:
160.1693
IUPAC Name:
4H-chromene-3-carbaldehyde
InChI:
InChI=1S/C10H8O2/c11-6-8-5-9-3-1-2-4-10(9)12-7-8/h1-4,6-7H,5H2
InChI Key:
CDTIRJYYEPOPNF-UHFFFAOYSA-N
SMILES:
O=CC1=COc2c(C1)cccc2
Properties
Complexity:
208
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
160.052g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
160.172g/mol
Monoisotopic Mass:
160.052g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
26.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.5
Literature
| Title | Journal |
|---|---|
| A domino Knoevenagel hetero-Diels-Alder reaction for the synthesis of polycyclic chromene derivatives and evaluation of their cytotoxicity. | Bioorganic & medicinal chemistry letters 20120301 |
| 3'-[Hy-droxy(4-oxo-4H-chromen-3-yl)meth-yl]-2-oxospiro-[indoline-3,2'-pyrrolidine]-3'-carbonitrile. | Acta crystallographica. Section E, Structure reports online 20120101 |
| Catalytic enantioselective domino oxa-michael/aldol condensations: asymmetric synthesis of benzopyran derivatives. | Chemistry (Weinheim an der Bergstrasse, Germany) 20070101 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.







