200,000+ products from a single source!
sales@angenechem.com
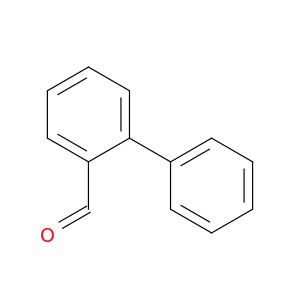
1203-68-5 | 2-Biphenylcarboxaldehyde
CAS No: 1203-68-5 Catalog No: AG00334O MDL No:MFCD01740431
Product Description
Catalog Number:
AG00334O
Chemical Name:
2-Biphenylcarboxaldehyde
CAS Number:
1203-68-5
Molecular Formula:
C13H10O
Molecular Weight:
182.2179
MDL Number:
MFCD01740431
IUPAC Name:
2-phenylbenzaldehyde
InChI:
InChI=1S/C13H10O/c14-10-12-8-4-5-9-13(12)11-6-2-1-3-7-11/h1-10H
InChI Key:
LCRCBXLHWTVPEQ-UHFFFAOYSA-N
SMILES:
O=Cc1ccccc1c1ccccc1
Properties
Complexity:
182
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
182.073g/mol
Formal Charge:
0
Heavy Atom Count:
14
Hydrogen Bond Acceptor Count:
1
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
182.222g/mol
Monoisotopic Mass:
182.073g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
17.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.4
Literature
| Title | Journal |
|---|---|
| Enantioselective synthesis of fluorene derivatives by chiral N-triflyl phosphoramide catalyzed double Friedel-Crafts alkylation reaction. | Organic & biomolecular chemistry 20120428 |
| Potent histone deacetylase inhibitors: N-hydroxybenzamides with antitumor activities. | Bioorganic & medicinal chemistry 20040815 |
| ERbeta ligands. Part 2: Synthesis and structure-activity relationships of a series of 4-hydroxy-biphenyl-carbaldehyde oxime derivatives. | Bioorganic & medicinal chemistry 20040515 |
| Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. | Journal of medicinal chemistry 20040115 |
| Highly enhanced enantioselectivity in the memory of chirality via acyliminium ions. | Organic letters 20020530 |
Related Products
© 2019 Angene International Limited. All rights Reserved.