200,000+ products from a single source!
sales@angenechem.com
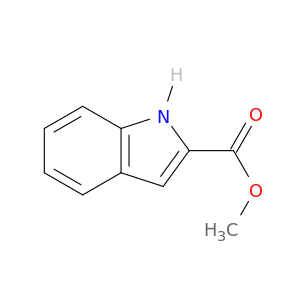
1202-04-6 | 1H-Indole-2-carboxylic acid, methyl ester
CAS No: 1202-04-6 Catalog No: AG000QK8 MDL No:MFCD00460779
Product Description
Catalog Number:
AG000QK8
Chemical Name:
1H-Indole-2-carboxylic acid, methyl ester
CAS Number:
1202-04-6
Molecular Formula:
C10H9NO2
Molecular Weight:
175.1840
MDL Number:
MFCD00460779
IUPAC Name:
methyl 1H-indole-2-carboxylate
InChI:
InChI=1S/C10H9NO2/c1-13-10(12)9-6-7-4-2-3-5-8(7)11-9/h2-6,11H,1H3
InChI Key:
NQPIEWBAWBFGOB-UHFFFAOYSA-N
SMILES:
COC(=O)c1cc2c([nH]1)cccc2
EC Number:
214-861-7
NSC Number:
78006
Properties
Complexity:
205
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
175.063g/mol
Formal Charge:
0
Heavy Atom Count:
13
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
175.187g/mol
Monoisotopic Mass:
175.063g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
42.1A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
2.5
Literature
| Title | Journal |
|---|---|
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Reactions of indole derivatives with oxiranes and aziridines on silica. Synthesis of beta-carbolin-1-one mimic of pancratistatin. | The Journal of organic chemistry 20050429 |
| New synthetic method for indole-2-carboxylate and its application to the total synthesis of duocarmycin SA. | Organic letters 20040819 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.