200,000+ products from a single source!
sales@angenechem.com
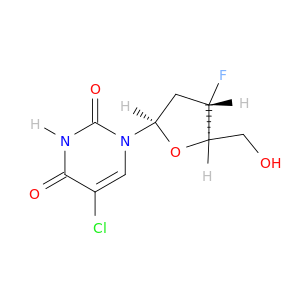
119644-22-3 | Uridine, 5-chloro-2',3'-dideoxy-3'-fluoro-
CAS No: 119644-22-3 Catalog No: AG000PDV MDL No:
Product Description
Catalog Number:
AG000PDV
Chemical Name:
Uridine, 5-chloro-2',3'-dideoxy-3'-fluoro-
CAS Number:
119644-22-3
Molecular Formula:
C9H10ClFN2O4
Molecular Weight:
264.6381
IUPAC Name:
5-chloro-1-[(2R,4S,5R)-4-fluoro-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione
InChI:
InChI=1S/C9H10ClFN2O4/c10-4-2-13(9(16)12-8(4)15)7-1-5(11)6(3-14)17-7/h2,5-7,14H,1,3H2,(H,12,15,16)/t5-,6+,7+/m0/s1
InChI Key:
WKVDSZYIGHLONN-RRKCRQDMSA-N
SMILES:
OC[C@H]1O[C@H](C[C@@H]1F)n1cc(Cl)c(=O)[nH]c1=O
UNII:
9D65NWY2K0
Properties
Complexity:
389
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
3
Defined Bond Stereocenter Count:
0
Exact Mass:
264.031g/mol
Formal Charge:
0
Heavy Atom Count:
17
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
264.637g/mol
Monoisotopic Mass:
264.031g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
78.9A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.2
Literature
| Title | Journal |
|---|---|
| Synthesis and in vitro anti-mycobacterial activity of 5-substituted pyrimidine nucleosides. | Bioorganic & medicinal chemistry 20051215 |
| Synthesis and antiviral evaluation of some beta-L-2', 3'-dideoxy-5-chloropyrimidine nucleosides and pronucleotides. | Antiviral research 20000301 |
| 5-Chloro-2',3'-dideoxy-3'-fluorouridine (935U83), a selective anti-human immunodeficiency virus agent with an improved metabolic and toxicological profile. | Antimicrobial agents and chemotherapy 19940701 |
| Use of a standardized cell culture assay to assess activities of nucleoside analogs against hepatitis B virus replication. | Antiviral research 19920701 |
| Synthesis and anti-HIV evaluation of 2',3'-dideoxyribo-5-chloropyrimidine analogues: reduced toxicity of 5-chlorinated 2',3'-dideoxynucleosides. | Journal of medicinal chemistry 19900601 |
| Inhibition of HIV-replication by 3'-fluoro-modified nucleosides with low cytotoxicity. | Biochemical and biophysical research communications 19891130 |
| 3'-Fluoro-2',3'-dideoxy-5-chlorouridine: most selective anti-HIV-1 agent among a series of new 2'- and 3'-fluorinated 2',3'-dideoxynucleoside analogues. | Journal of medicinal chemistry 19890801 |
| 5-Halogeno-3'-fluoro-2',3'-dideoxyuridines as inhibitors of human immunodeficiency virus (HIV): potent and selective anti-HIV activity of 3'-fluoro-2',3'-dideoxy-5-chlorouridine. | Molecular pharmacology 19890501 |
| 5-Chloro-substituted derivatives of 2', 3'-didehydro-2',3'-dideoxyuridine, 3'-fluoro-2',3'-dideoxyuridine and 3'-azido-2',3'-dideoxyuridine as anti-HIV agents. | Biochemical pharmacology 19890315 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.







