200,000+ products from a single source!
sales@angenechem.com
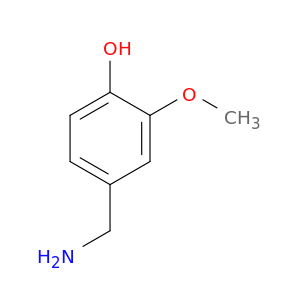
1196-92-5 | Phenol, 4-(aminomethyl)-2-methoxy-
CAS No: 1196-92-5 Catalog No: AG000P93 MDL No:MFCD00044577
Product Description
Catalog Number:
AG000P93
Chemical Name:
Phenol, 4-(aminomethyl)-2-methoxy-
CAS Number:
1196-92-5
Molecular Formula:
C8H11NO2
Molecular Weight:
153.1784
MDL Number:
MFCD00044577
IUPAC Name:
4-(aminomethyl)-2-methoxyphenol
InChI:
InChI=1S/C8H11NO2/c1-11-8-4-6(5-9)2-3-7(8)10/h2-4,10H,5,9H2,1H3
InChI Key:
WRPWWVNUCXQDQV-UHFFFAOYSA-N
SMILES:
COc1cc(CN)ccc1O
EC Number:
422-450-0
UNII:
1WEZ91E3Z0
Properties
Complexity:
119
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
153.079g/mol
Formal Charge:
0
Heavy Atom Count:
11
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
153.181g/mol
Monoisotopic Mass:
153.079g/mol
Rotatable Bond Count:
2
Topological Polar Surface Area:
55.5A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-0.2
Literature
| Title | Journal |
|---|---|
| Functional validation of Capsicum frutescens aminotransferase gene involved in vanillylamine biosynthesis using Agrobacterium mediated genetic transformation studies in Nicotiana tabacum and Capsicum frutescens calli cultures. | Plant science : an international journal of experimental plant biology 20121001 |
| Synthesis of stable isotope-labeled precursors for the biosyntheses of capsaicinoids, capsinoids, and capsiconinoids. | Bioscience, biotechnology, and biochemistry 20110101 |
| Understanding and classifying metabolite space and metabolite-likeness. | PloS one 20110101 |
| Influence of 'remote' intramolecular hydrogen bonds on the stabilities of phenoxyl radicals and benzyl cations. | The Journal of organic chemistry 20100702 |
| Functional loss of pAMT results in biosynthesis of capsinoids, capsaicinoid analogs, in Capsicum annuum cv. CH-19 Sweet. | The Plant journal : for cell and molecular biology 20090901 |
| In vitro hepatic and skin metabolism of capsaicin. | Drug metabolism and disposition: the biological fate of chemicals 20080401 |
| Enzymatic synthesis of capsaicin analogs and their effect on the T-type Ca2+ channels. | Biochemical and biophysical research communications 20070504 |
| Genetic control of pungency in C. chinense via the Pun1 locus. | Journal of experimental botany 20070101 |
| Valine pathway is more crucial than phenyl propanoid pathway in regulating capsaicin biosynthesis in Capsicum frutescens mill. | Journal of agricultural and food chemistry 20060906 |
| Capsinoid is biosynthesized from phenylalanine and valine in a non-pungent pepper, Capsicum annuum L. cv. CH-19 sweet. | Bioscience, biotechnology, and biochemistry 20060601 |
| Influence of 8-methyl-nonenoic acid on capsaicin biosynthesis in in-vivo and in-vitro cell cultures of Capsicum spp. | Journal of agricultural and food chemistry 20060308 |
| Utilization of capsaicin and vanillylamine as growth substrates by Capsicum (hot pepper)-associated bacteria. | Environmental microbiology 20060301 |
| Apoptosis induction by dohevanil, a DHA substitutive analog of capsaicin, in MCF-7 cells. | Life sciences 20060223 |
| A novel acylase from Streptomyces mobaraensis that efficiently catalyzes hydrolysis/synthesis of capsaicins as well as N-acyl-L-amino acids and N-acyl-peptides. | Journal of agricultural and food chemistry 20060111 |
| The Pun1 gene for pungency in pepper encodes a putative acyltransferase. | The Plant journal : for cell and molecular biology 20050601 |
| Methyl jasmonate modulated biotransformation of phenylpropanoids to vanillin related metabolites using Capsicum frutescens root cultures. | Plant physiology and biochemistry : PPB 20050201 |
| Quantification of unconjugated metanephrines in human plasma without interference by acetaminophen. | Clinical chemistry 20010601 |
| Enzymatic synthesis of vanillin. | Journal of agricultural and food chemistry 20010601 |
Related Products
© 2019 Angene International Limited. All rights Reserved.