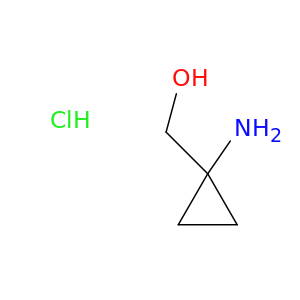
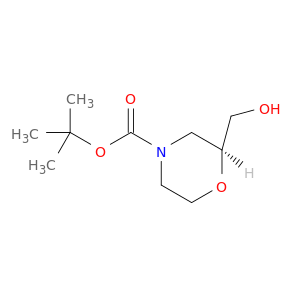
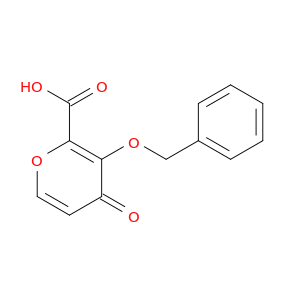
200,000+ products from a single source!
sales@angenechem.com
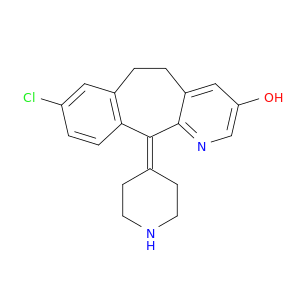
119410-08-1 | 5H-Benzo[5,6]cyclohepta[1,2-b]pyridin-3-ol, 8-chloro-6,11-dihydro-11-(4-piperidinylidene)-
CAS No: 119410-08-1 Catalog No: AG000IR2 MDL No:
Product Description
Catalog Number:
AG000IR2
Chemical Name:
5H-Benzo[5,6]cyclohepta[1,2-b]pyridin-3-ol, 8-chloro-6,11-dihydro-11-(4-piperidinylidene)-
CAS Number:
119410-08-1
Molecular Formula:
C19H19ClN2O
Molecular Weight:
326.8200
IUPAC Name:
13-chloro-2-piperidin-4-ylidene-4-azatricyclo[9.4.0.03,8]pentadeca-1(11),3(8),4,6,12,14-hexaen-6-ol
InChI:
InChI=1S/C19H19ClN2O/c20-15-3-4-17-13(9-15)1-2-14-10-16(23)11-22-19(14)18(17)12-5-7-21-8-6-12/h3-4,9-11,21,23H,1-2,5-8H2
InChI Key:
NDFMTPISBHBIKE-UHFFFAOYSA-N
SMILES:
Oc1cnc2c(c1)CCc1c(C2=C2CCNCC2)ccc(c1)Cl
UNII:
3H9FFN759V
Properties
Complexity:
458
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
326.119g/mol
Formal Charge:
0
Heavy Atom Count:
23
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
326.824g/mol
Monoisotopic Mass:
326.119g/mol
Rotatable Bond Count:
0
Topological Polar Surface Area:
45.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
4.1
Literature
| Title | Journal |
|---|---|
| Prevalence of desloratadine poor metabolizer phenotype in healthy Jordanian males. | Biopharmaceutics & drug disposition 20120101 |
| Pharmacokinetic and safety profile of rupatadine when coadministered with azithromycin at steady-state levels: a randomized, open-label, two-way, crossover, Phase I study. | Clinical therapeutics 20080901 |
| Simultaneous determination of desloratadine and its active metabolite 3-hydroxydesloratadine in human plasma by LC/MS/MS and its application to pharmacokinetics and bioequivalence. | Journal of pharmaceutical and biomedical analysis 20071130 |
| Influence of food on the oral bioavailability of rupatadine tablets in healthy volunteers: a single-dose, randomized, open-label, two-way crossover study. | Clinical therapeutics 20070501 |
| Orthogonal extraction/chromatography and UPLC, two powerful new techniques for bioanalytical quantitation of desloratadine and 3-hydroxydesloratadine at 25 pg/mL. | Journal of pharmaceutical and biomedical analysis 20060224 |
| Pharmacokinetics/pharmacodynamics of desloratadine and fluoxetine in healthy volunteers. | Journal of clinical pharmacology 20041101 |
| Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of 3-hydroxydesloratadine. | Biopharmaceutics & drug disposition 20040901 |
| Validation of a sensitive and automated 96-well solid-phase extraction liquid chromatography-tandem mass spectrometry method for the determination of desloratadine and 3-hydroxydesloratadine in human plasma. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20030725 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.