200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1193-79-9
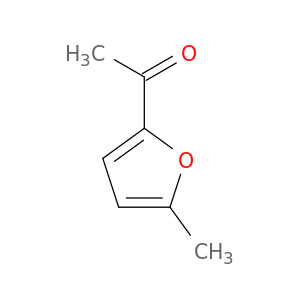
1193-79-9 | Ethanone, 1-(5-methyl-2-furanyl)-
CAS No: 1193-79-9 Catalog No: AG000IJS MDL No:MFCD00003243
Product Description
Catalog Number:
AG000IJS
Chemical Name:
Ethanone, 1-(5-methyl-2-furanyl)-
CAS Number:
1193-79-9
Molecular Formula:
C7H8O2
Molecular Weight:
124.1372
MDL Number:
MFCD00003243
IUPAC Name:
1-(5-methylfuran-2-yl)ethanone
InChI:
InChI=1S/C7H8O2/c1-5-3-4-7(9-5)6(2)8/h3-4H,1-2H3
InChI Key:
KEFJLCGVTHRGAH-UHFFFAOYSA-N
SMILES:
Cc1ccc(o1)C(=O)C
EC Number:
214-779-1
UNII:
IY49408H2O
NSC Number:
80404
FEMA Number:
3609
Properties
Complexity:
120
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
124.052g/mol
Formal Charge:
0
Heavy Atom Count:
9
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
0
Isotope Atom Count:
0
Molecular Weight:
124.139g/mol
Monoisotopic Mass:
124.052g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
30.2A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
1.4
Literature
| Title | Journal |
|---|---|
| Thermal decomposition of 5-(hydroxymethyl)-2-furaldehyde (HMF) and its further transformations in the presence of glycine. | Journal of agricultural and food chemistry 20110928 |
| Use of phosphonyl carbanions in the synthesis of anti-inflammatory active phosphorus-containing fused heterocycles and relevance phosphonates. | European journal of medicinal chemistry 20101101 |
| Investigation of trypanothione reductase as a drug target in Trypanosoma brucei. | ChemMedChem 20091207 |
| Monitoring cytotoxic potentials of furfuryl alcohol and 2-furyl methyl ketone in mice. | Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 20080101 |
| Antitubercular constituents from the stem wood of Cinnamomum kotoense. | Journal of natural products 20050901 |
| Aging of proteins: immunological detection of a glucose-derived pyrrole formed during maillard reaction in vivo. | The Journal of biological chemistry 19890305 |
Related Products
© 2019 Angene International Limited. All rights Reserved.