200,000+ products from a single source!
sales@angenechem.com
Home > Aldehydes > 118354-84-0
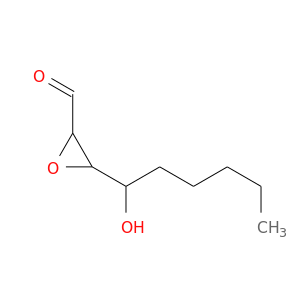
118354-84-0 | 2-Oxiranecarboxaldehyde,3-(1-hydroxyhexyl)-
CAS No: 118354-84-0 Catalog No: AG00817I MDL No:
Product Description
Catalog Number:
AG00817I
Chemical Name:
2-Oxiranecarboxaldehyde,3-(1-hydroxyhexyl)-
CAS Number:
118354-84-0
Molecular Formula:
C9H16O3
Molecular Weight:
172.2215
IUPAC Name:
3-(1-hydroxyhexyl)oxirane-2-carbaldehyde
InChI:
InChI=1S/C9H16O3/c1-2-3-4-5-7(11)9-8(6-10)12-9/h6-9,11H,2-5H2,1H3
InChI Key:
RWEZZEBPLLEJBN-UHFFFAOYSA-N
SMILES:
CCCCCC(C1OC1C=O)O
Properties
Complexity:
147
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
172.11g/mol
Formal Charge:
0
Heavy Atom Count:
12
Hydrogen Bond Acceptor Count:
3
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
172.224g/mol
Monoisotopic Mass:
172.11g/mol
Rotatable Bond Count:
6
Topological Polar Surface Area:
49.8A^2
Undefined Atom Stereocenter Count:
3
Undefined Bond Stereocenter Count:
0
XLogP3:
1.2
Literature
| Title | Journal |
|---|---|
| Formation of a N2-dG:N2-dG carbinolamine DNA cross-link by the trans-4-hydroxynonenal-derived (6S,8R,11S) 1,N2-dG adduct. | Journal of the American Chemical Society 20111012 |
| Synthesis of the four stereoisomers of 2,3-epoxy-4-hydroxynonanal and their reactivity with deoxyguanosine. | Organic & biomolecular chemistry 20110321 |
| Nucleotide excision repair and recombination are engaged in repair of trans-4-hydroxy-2-nonenal adducts to DNA bases in Escherichia coli. | International journal of biological sciences 20090101 |
| Quenching of alpha,beta-unsaturated aldehydes by green tea polyphenols: HPLC-ESI-MS/MS studies. | Journal of pharmaceutical and biomedical analysis 20081104 |
| The stereochemistry of trans-4-hydroxynonenal-derived exocyclic 1,N2-2'-deoxyguanosine adducts modulates formation of interstrand cross-links in the 5'-CpG-3' sequence. | Biochemistry 20081104 |
| Rearrangement of the (6S,8R,11S) and (6R,8S,11R) exocyclic 1,N2-deoxyguanosine adducts of trans-4-hydroxynonenal to N2-deoxyguanosine cyclic hemiacetal adducts when placed complementary to cytosine in duplex DNA. | Journal of the American Chemical Society 20080820 |
| 4-Hydroperoxy-2-nonenal-induced formation of 1,N2-etheno-2'-deoxyguanosine adducts. | Chemical research in toxicology 20050401 |
| Analysis of ethenoguanine adducts in human urine using high performance liquid chromatography-tandem mass spectrometry. | Toxicology letters 20020805 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.







