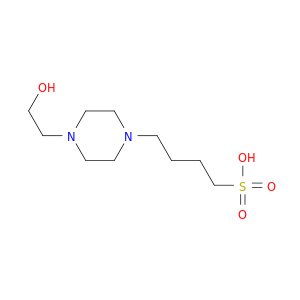
200,000+ products from a single source!
sales@angenechem.com
Home > Imidazoles > 117977-21-6
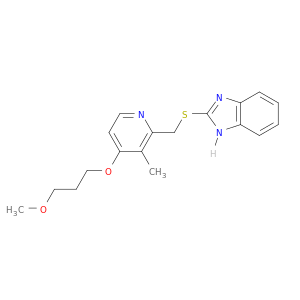
117977-21-6 | 2-[[[4-(3-Methoxypropoxy)-3-methylpyridine-2-yl ]methyl]thio]-1H-benzimidazole
CAS No: 117977-21-6 Catalog No: AG007QM8 MDL No:MFCD08063845
Product Description
Catalog Number:
AG007QM8
Chemical Name:
2-[[[4-(3-Methoxypropoxy)-3-methylpyridine-2-yl ]methyl]thio]-1H-benzimidazole
CAS Number:
117977-21-6
Molecular Formula:
C18H21N3O2S
Molecular Weight:
343.4432
MDL Number:
MFCD08063845
IUPAC Name:
2-[[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methylsulfanyl]-1H-benzimidazole
InChI:
InChI=1S/C18H21N3O2S/c1-13-16(19-9-8-17(13)23-11-5-10-22-2)12-24-18-20-14-6-3-4-7-15(14)21-18/h3-4,6-9H,5,10-12H2,1-2H3,(H,20,21)
InChI Key:
BSXAHDOWMOSVAP-UHFFFAOYSA-N
SMILES:
COCCCOc1ccnc(c1C)CSc1nc2c([nH]1)cccc2
UNII:
304S7E320P
Properties
Complexity:
374
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
343.135g/mol
Formal Charge:
0
Heavy Atom Count:
24
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
343.445g/mol
Monoisotopic Mass:
343.135g/mol
Rotatable Bond Count:
8
Topological Polar Surface Area:
85.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.3
Literature
| Title | Journal |
|---|---|
| Randomized, open-label, single-dose, crossover, relative bioavailability study in healthy adults, comparing the pharmacokinetics of rabeprazole granules administered using soft food or infant formula as dosing vehicle versus suspension. | Clinical therapeutics 20120701 |
| In vitro metabolic stability of moisture-sensitive rabeprazole in human liver microsomes and its modulation by pharmaceutical excipients. | Archives of pharmacal research 20080301 |
| Identification and characterization of potential impurities of rabeprazole sodium. | Journal of pharmaceutical and biomedical analysis 20070312 |
| Identification of the time-point which gives a plasma rabeprazole concentration that adequately reflects the area under the concentration-time curve. | European journal of clinical pharmacology 20061001 |
| Stereoselective metabolism of rabeprazole-thioether to rabeprazole by human liver microsomes. | European journal of clinical pharmacology 20060201 |
| Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. | Drug metabolism and disposition: the biological fate of chemicals 20040801 |
Related Products
Featured Products
© 2019 Angene International Limited. All rights Reserved.