200,000+ products from a single source!
sales@angenechem.com
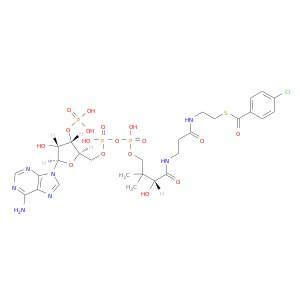
117380-98-0 | Coenzyme A, S-(4-chlorobenzoate)
CAS No: 117380-98-0 Catalog No: AG000E72 MDL No:
Product Description
Catalog Number:
AG000E72
Chemical Name:
Coenzyme A, S-(4-chlorobenzoate)
CAS Number:
117380-98-0
Molecular Formula:
C28H39ClN7O17P3S
Molecular Weight:
906.0852
IUPAC Name:
S-[2-[3-[[(2R)-4-[[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-2-hydroxy-3,3-dimethylbutanoyl]amino]propanoylamino]ethyl] 4-chlorobenzenecarbothioate
InChI:
InChI=1S/C28H39ClN7O17P3S/c1-28(2,22(39)25(40)32-8-7-18(37)31-9-10-57-27(41)15-3-5-16(29)6-4-15)12-50-56(47,48)53-55(45,46)49-11-17-21(52-54(42,43)44)20(38)26(51-17)36-14-35-19-23(30)33-13-34-24(19)36/h3-6,13-14,17,20-22,26,38-39H,7-12H2,1-2H3,(H,31,37)(H,32,40)(H,45,46)(H,47,48)(H2,30,33,34)(H2,42,43,44)/t17-,20-,21-,22+,26-/m1/s1
InChI Key:
DEPSOKCZMQPCBI-TYHXJLICSA-N
SMILES:
O=C(NCCSC(=O)c1ccc(cc1)Cl)CCNC(=O)[C@@H](C(COP(=O)(OP(=O)(OC[C@H]1O[C@H]([C@@H]([C@@H]1OP(=O)(O)O)O)n1cnc2c1ncnc2N)O)O)(C)C)O
Properties
Complexity:
1550
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
5
Defined Bond Stereocenter Count:
0
Exact Mass:
905.102g/mol
Formal Charge:
0
Heavy Atom Count:
57
Hydrogen Bond Acceptor Count:
22
Hydrogen Bond Donor Count:
9
Isotope Atom Count:
0
Molecular Weight:
906.083g/mol
Monoisotopic Mass:
905.102g/mol
Rotatable Bond Count:
21
Topological Polar Surface Area:
389A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-3.4
Literature
| Title | Journal |
|---|---|
| QM/MM studies of the enzyme-catalyzed dechlorination of 4-chlorobenzoyl-CoA provide insight into reaction energetics. | Journal of the American Chemical Society 20041027 |
| The strength of dehalogenase-substrate hydrogen bonding correlates with the rate of Meisenheimer intermediate formation. | Biochemistry 20030812 |
| Raman evidence for Meisenheimer complex formation in the hydrolysis reactions of 4-fluorobenzoyl- and 4-nitrobenzoyl-coenzyme A catalyzed by 4-chlorobenzoyl-coenzyme A dehalogenase. | Biochemistry 20020611 |
| Role of active site binding interactions in 4-chlorobenzoyl-coenzyme A dehalogenase catalysis. | Biochemistry 20011225 |
| Histidine 90 function in 4-chlorobenzoyl-coenzyme a dehalogenase catalysis. | Biochemistry 20011113 |
Related Products
© 2019 Angene International Limited. All rights Reserved.