200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 116297-02-0
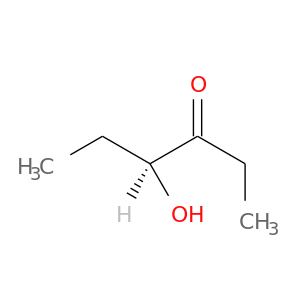
116297-02-0 | 3-Hexanone, 4-hydroxy-, (4S)-
CAS No: 116297-02-0 Catalog No: AG000C5I MDL No:
Product Description
Catalog Number:
AG000C5I
Chemical Name:
3-Hexanone, 4-hydroxy-, (4S)-
CAS Number:
116297-02-0
Molecular Formula:
C6H12O2
Molecular Weight:
116.1583
IUPAC Name:
4-hydroxyhexan-3-one
InChI:
InChI=1S/C6H12O2/c1-3-5(7)6(8)4-2/h5,7H,3-4H2,1-2H3
InChI Key:
SKCYVGUCBRYGTE-UHFFFAOYSA-N
SMILES:
CCC(=O)[C@H](CC)O
EC Number:
225-637-3
NSC Number:
23087
Properties
Complexity:
78.6
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
0
Defined Bond Stereocenter Count:
0
Exact Mass:
116.084g/mol
Formal Charge:
0
Heavy Atom Count:
8
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
116.16g/mol
Monoisotopic Mass:
116.084g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
37.3A^2
Undefined Atom Stereocenter Count:
1
Undefined Bond Stereocenter Count:
0
XLogP3:
0.7
Literature
| Title | Journal |
|---|---|
| Biocatalytic production of alpha-hydroxy ketones and vicinal diols by yeast and human aldo-keto reductases. | Chemico-biological interactions 20130225 |
| Mechanism of acetaldehyde-induced deactivation of microbial lipases. | BMC biochemistry 20110101 |
| Direct spectrophotometric assay for benzaldehyde lyase activity. | Biotechnology research international 20110101 |
| Propioin synthesis using thiamine diphosphate-dependent enzymes. | Biotechnology progress 20090101 |
| An activity, stability and selectivity comparison of propioin synthesis by thiamine diphosphate-dependent enzymes in a solid/gas bioreactor. | Chembiochem : a European journal of chemical biology 20070618 |
Related Products
© 2019 Angene International Limited. All rights Reserved.