200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1162-53-4
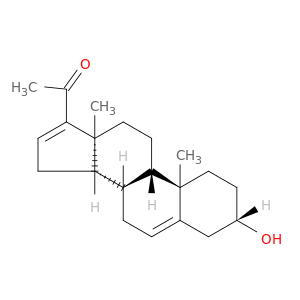
1162-53-4 | Pregna-5,16-dien-20-one, 3-hydroxy-, (3β)-
CAS No: 1162-53-4 Catalog No: AG000BW3 MDL No:MFCD00046219
Product Description
Catalog Number:
AG000BW3
Chemical Name:
Pregna-5,16-dien-20-one, 3-hydroxy-, (3β)-
CAS Number:
1162-53-4
Molecular Formula:
C21H30O2
Molecular Weight:
314.4617
MDL Number:
MFCD00046219
IUPAC Name:
1-[(3S,8R,9S,10R,13S,14S)-3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15-decahydro-1H-cyclopenta[a]phenanthren-17-yl]ethanone
InChI:
InChI=1S/C21H30O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4,6,15-16,18-19,23H,5,7-12H2,1-3H3/t15-,16-,18-,19-,20-,21+/m0/s1
InChI Key:
YLFRRPUBVUAHSR-RRPFGEQOSA-N
SMILES:
O[C@H]1CC[C@]2(C(=CC[C@@H]3[C@@H]2CC[C@]2([C@H]3CC=C2C(=O)C)C)C1)C
EC Number:
214-602-8
UNII:
7349506P5S
NSC Number:
15467
Properties
Complexity:
601
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
6
Defined Bond Stereocenter Count:
0
Exact Mass:
314.225g/mol
Formal Charge:
0
Heavy Atom Count:
23
Hydrogen Bond Acceptor Count:
2
Hydrogen Bond Donor Count:
1
Isotope Atom Count:
0
Molecular Weight:
314.469g/mol
Monoisotopic Mass:
314.225g/mol
Rotatable Bond Count:
1
Topological Polar Surface Area:
37.3A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
3.9
Literature
| Title | Journal |
|---|---|
| Dose escalation pharmacokinetics and lipid lowering activity of a novel farnesoid X receptor modulator: 16-Dehydropregnenolone. | Indian journal of pharmacology 20120101 |
| A Validated Reverse Phase HPLC Analytical Method for Quantitation of Glycoalkaloids in Solanum lycocarpum and Its Extracts. | Journal of analytical methods in chemistry 20120101 |
| Activation of ATM-Chk2 by 16-dehydropregnenolone induces G1 phase arrest and apoptosis in HeLa cells. | Journal of Asian natural products research 20120101 |
| Aza-annulation on the 16-dehydropregnenolone, via tandem intermolecular aldol process and intramolecular Michael addition. | Bioorganic & medicinal chemistry letters 20110415 |
| Preclinical pharmacokinetics, dose proportionality, gender difference and protein binding study of 16-dehydropregnenolone, an antihyperlipidemic agent, in rats. | The Journal of pharmacy and pharmacology 20110101 |
| In vivo and in vitro effect of novel 4,16-pregnadiene-6,20-dione derivatives, as 5alpha-reductase inhibitors. | The Journal of steroid biochemistry and molecular biology 20080901 |
| Cytotoxic constituents from Solanum lyratum. | Archives of pharmacal research 20060201 |
| A sensitive and selective HPLC/ESI-MS/MS assay for the simultaneous quantification of 16-dehydropregnenolone and its major metabolites in rabbit plasma. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20060102 |
| Synthesis of some new steroidal [16alpha,17alpha-d]-isoxazolines. | Steroids 20050701 |
| HPLC-UV method development and validation for 16-dehydropregnenolone, a novel oral hypolipidaemic agent, in rat biological matrices for application to pharmacokinetic studies. | Journal of pharmaceutical and biomedical analysis 20031124 |
| Development of an enzyme immunoassay for serum 16-dehydropregnenolone. | Biological & pharmaceutical bulletin 20010801 |
Related Products
© 2019 Angene International Limited. All rights Reserved.