200,000+ products from a single source!
sales@angenechem.com
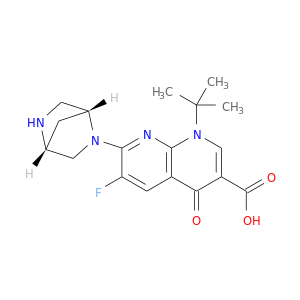
116143-32-9 | 1,8-Naphthyridine-3-carboxylic acid, 7-(1R,4R)-2,5-diazabicyclo[2.2.1]hept-2-yl-1-(1,1-dimethylethyl)-6-fluoro-1,4-dihydro-4-oxo-
CAS No: 116143-32-9 Catalog No: AG000BR2 MDL No:
Product Description
Catalog Number:
AG000BR2
Chemical Name:
1,8-Naphthyridine-3-carboxylic acid, 7-(1R,4R)-2,5-diazabicyclo[2.2.1]hept-2-yl-1-(1,1-dimethylethyl)-6-fluoro-1,4-dihydro-4-oxo-
CAS Number:
116143-32-9
Molecular Formula:
C18H21FN4O3
Molecular Weight:
360.3827
IUPAC Name:
1-tert-butyl-7-[(1R,4R)-2,5-diazabicyclo[2.2.1]heptan-2-yl]-6-fluoro-4-oxo-1,8-naphthyridine-3-carboxylic acid
InChI:
InChI=1S/C18H21FN4O3/c1-18(2,3)23-8-12(17(25)26)14(24)11-5-13(19)16(21-15(11)23)22-7-9-4-10(22)6-20-9/h5,8-10,20H,4,6-7H2,1-3H3,(H,25,26)/t9-,10-/m1/s1
InChI Key:
KNHLHFDFFDCJJR-NXEZZACHSA-N
SMILES:
Fc1cc2c(nc1N1C[C@H]3C[C@@H]1CN3)n(cc(c2=O)C(=O)O)C(C)(C)C
Properties
Complexity:
661
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
2
Defined Bond Stereocenter Count:
0
Exact Mass:
360.16g/mol
Formal Charge:
0
Heavy Atom Count:
26
Hydrogen Bond Acceptor Count:
8
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
360.389g/mol
Monoisotopic Mass:
360.16g/mol
Rotatable Bond Count:
3
Topological Polar Surface Area:
85.8A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.7
Literature
| Title | Journal |
|---|---|
| Fluoronaphthyridines as antibacterial agents. 6. Synthesis and structure-activity relationships of new chiral 7-(1-, 3-, 4-, and 6-methyl-2,5-diazabicyclo[2.2.1]heptan-2-yl)naphthyridine analogues of 7-[(1R,4R)-2,5- diazabicyclo[2.2.1]heptan-2-yl]-1-(1,1-dimethylethyl)-6-fluoro-1,4-dihy dro-4-oxo-1,8-naphthyridine-3-carboxylic acid. Influence of the configuration on blood pressure in dogs. A quinolone-class effect. | Journal of medicinal chemistry 19920724 |
| Fluoronaphthyridines and -quinolones as antibacterial agents. 3. Synthesis and structure-activity relationships of new 1-(1,1-dimethyl-2-fluoroethyl), 1-[1-methyl-1-(fluoromethyl)-2-fluoroethyl], and 1-[1,1-(difluoromethyl)-2-fluoroethyl] substituted derivatives. | Journal of medicinal chemistry 19910101 |
| Comparative in vitro activity of the new fluoroquinolone BMY-40062. | European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology 19900801 |
| Fluoronaphthyridines and quinolones as antibacterial agents. 2. Synthesis and structure-activity relationships of new 1-tert-butyl 7-substituted derivatives. | Journal of medicinal chemistry 19900501 |
| In vitro and in vivo antibacterial activities of BMY 40062, a new fluoronaphthyridone. | Antimicrobial agents and chemotherapy 19890601 |
Related Products
© 2019 Angene International Limited. All rights Reserved.