200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1160-54-9
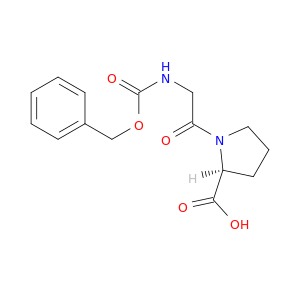
1160-54-9 | L-Proline, N-[(phenylmethoxy)carbonyl]glycyl-
CAS No: 1160-54-9 Catalog No: AG000BDC MDL No:MFCD00037341
Product Description
Catalog Number:
AG000BDC
Chemical Name:
L-Proline, N-[(phenylmethoxy)carbonyl]glycyl-
CAS Number:
1160-54-9
Molecular Formula:
C15H18N2O5
Molecular Weight:
306.3138
MDL Number:
MFCD00037341
IUPAC Name:
(2S)-1-[2-(phenylmethoxycarbonylamino)acetyl]pyrrolidine-2-carboxylic acid
InChI:
InChI=1S/C15H18N2O5/c18-13(17-8-4-7-12(17)14(19)20)9-16-15(21)22-10-11-5-2-1-3-6-11/h1-3,5-6,12H,4,7-10H2,(H,16,21)(H,19,20)/t12-/m0/s1
InChI Key:
ZTUKZKYDJMGJDC-LBPRGKRZSA-N
SMILES:
O=C(OCc1ccccc1)NCC(=O)N1CCC[C@H]1C(=O)O
EC Number:
214-598-8
Properties
Complexity:
420
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
306.122g/mol
Formal Charge:
0
Heavy Atom Count:
22
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
2
Isotope Atom Count:
0
Molecular Weight:
306.318g/mol
Monoisotopic Mass:
306.122g/mol
Rotatable Bond Count:
6
Topological Polar Surface Area:
95.9A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
0.7
Literature
| Title | Journal |
|---|---|
| Synthesis and neuroprotective activity of analogues of glycyl-L-prolyl-L-glutamic acid (GPE) modified at the alpha-carboxylic acid. | Bioorganic & medicinal chemistry 20050117 |
| Synthesis and pharmacological evaluation of side chain modified glutamic acid analogues of the neuroprotective agent glycyl-L-prolyl-L-glutamic acid (GPE). | Bioorganic & medicinal chemistry 20050117 |
| Chiral separation of amines with N-benzoxycarbonylglycyl-L-proline as selector in non-aqueous capillary electrophoresis using methanol and 1,2-dichloroethane in the background electrolyte. | Journal of chromatography. A 20030117 |
| Structures of prolyl oligopeptidase substrate/inhibitor complexes. Use of inhibitor binding for titration of the catalytic histidine residue. | The Journal of biological chemistry 20010112 |
Related Products
© 2019 Angene International Limited. All rights Reserved.