200,000+ products from a single source!
sales@angenechem.com
Home > Other Building Blocks > 1155-64-2
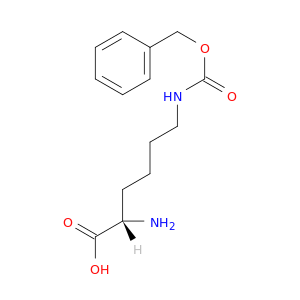
1155-64-2 | H-Lys(Z)-OH
CAS No: 1155-64-2 Catalog No: AG003SO6 MDL No:MFCD00002638
Product Description
Catalog Number:
AG003SO6
Chemical Name:
H-Lys(Z)-OH
CAS Number:
1155-64-2
Molecular Formula:
C14H20N2O4
Molecular Weight:
280.3196
MDL Number:
MFCD00002638
IUPAC Name:
(2S)-2-amino-6-(phenylmethoxycarbonylamino)hexanoic acid
InChI:
InChI=1S/C14H20N2O4/c15-12(13(17)18)8-4-5-9-16-14(19)20-10-11-6-2-1-3-7-11/h1-3,6-7,12H,4-5,8-10,15H2,(H,16,19)(H,17,18)/t12-/m0/s1
InChI Key:
CKGCFBNYQJDIGS-LBPRGKRZSA-N
SMILES:
O=C(OCc1ccccc1)NCCCC[C@@H](C(=O)O)N
EC Number:
214-585-7
Properties
Complexity:
304
Compound Is Canonicalized:
Yes
Covalently-Bonded Unit Count:
1
Defined Atom Stereocenter Count:
1
Defined Bond Stereocenter Count:
0
Exact Mass:
280.142g/mol
Formal Charge:
0
Heavy Atom Count:
20
Hydrogen Bond Acceptor Count:
5
Hydrogen Bond Donor Count:
3
Isotope Atom Count:
0
Molecular Weight:
280.324g/mol
Monoisotopic Mass:
280.142g/mol
Rotatable Bond Count:
9
Topological Polar Surface Area:
102A^2
Undefined Atom Stereocenter Count:
0
Undefined Bond Stereocenter Count:
0
XLogP3:
-1.1
Literature
| Title | Journal |
|---|---|
| Wide-range protein photo-crosslinking achieved by a genetically encoded N(ε)-(benzyloxycarbonyl)lysine derivative with a diazirinyl moiety. | Molecular bioSystems 20120401 |
| Multistep engineering of pyrrolysyl-tRNA synthetase to genetically encode N(epsilon)-(o-azidobenzyloxycarbonyl) lysine for site-specific protein modification. | Chemistry & biology 20081124 |
| Adding l-lysine derivatives to the genetic code of mammalian cells with engineered pyrrolysyl-tRNA synthetases. | Biochemical and biophysical research communications 20080711 |
| Novel amphiphilic poly(epsilon-caprolactone)-g-poly(L-lysine) degradable copolymers. | Biomacromolecules 20070801 |
| Synthesis of Nepsilon-protected-L-lysine and gamma-benzyl-L-glutamate N-carboxyanhydrides (NCA) by carbamoylation and nitrosation. | Amino acids 20041001 |
| Formation of a two-dimensionally well-ordered monolayer of a peptide oligomer by a simple spin-coating process. | Langmuir : the ACS journal of surfaces and colloids 20040203 |
Related Products
© 2019 Angene International Limited. All rights Reserved.